
Exams Prep Master
Graphite originated from the Greek term graphene, which means "writing," and its name comes from the fact that it was originally used to make pencils. It is a carbon aloe trope that is grey in colour and opaque. It is recovered from the earth, but its formation takes years. In the meanwhile, the highest source of graphite is in China. As it is made up of carbon atoms, graphite is a non-metal. One of its properties, however, causes it to behave like a metal. Graphite is favoured by high pressure and high temperature, and it is the most stable form of carbon. Diamond will tend to change to graphite under these normal circumstances.
| Table of Content |
Key Terms: Graphite, crystal carbon, carbon compounds, carbon atom, covalent bonds.
Also check: Versatile nature of carbon
What is Graphite?
[Click Here for Sample Questions]
Graphite is a half-metal and a kind of crystal carbon, as well as one of the most well-known carbon allotropes. It would be among the most robust forms of carbon accessible under perfect conditions. To establish a standard temperature for the production of carbon compounds. However, because the process requires millions of years, graphite is a significantly more durable form of carbon than diamond under these regular standard settings. Yeah. If it is burned in the open air, carbon dioxide gas is produced. Aqua dog's first stop refers to a graphite aqueous solution. Plumbago is another name for graphite.

With a density of 2.09–2.23 g/cm3, graphite is an excellent heat and electrical conductor. Edward G. Acheson accidentally produced graphite for the first time while working on a high-temperature carborundum experiment. He discovered that at roughly 4150oC, the silicon in carborundum vaporises, leaving the carbon in graphite form behind. In 1896, he was given a patent for graphite fabrication, and commercial production of the material began in 1897. Graphite is a carbon allotrope, neither an element nor a combination. It doesn't have its own chemical formula.
Structure of Graphite
[Click Here for Sample Questions]
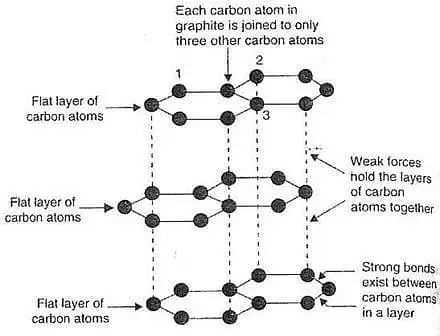
Graphite is a carbon allotrope. Each carbon atom in graphite is joined to three other carbon atoms by a single covalent bond, forming a hexagonal ring that is organized in a layer. In general, graphite is employed as an electrode in pencil batteries. It has a structure that is made up of two-dimensional layers. A sandwich-like structure is another name for it. Each carbon atom is SP to hybridize in graphite's structure, and hexagons are grouped in layers. Each carbon atom in the layer is bonded to three other carbon atoms in the same plane via covalent bonds. Weak wall forces between two layers satisfy the 4th valence of the carbon atom.
Properties of Graphite
[Click Here for Sample Questions]
Graphite is a carbon allotrope that is used in nuclear power reactors to make moderator rods. The following are its characteristics:
- Graphite can be found in its natural condition or manufactured artificially.
- It's an opaque greyish black material.
- Smooth and slippery to the touch, lighter than diamond.
- It is a good heat and electrical conductor.
- The sp2 hybridization of carbon atoms occurs.
- It's a crystalline substance.
- It melts at around 1800 degrees Fahrenheit.
- Non-inflammable.
- Vander waal forces are weak, therefore it's soft.
Also read: Organic compounds
Types of Graphite
[Click Here for Sample Questions]
- Natural Graphite
Natural graphite is a graphitic carbon mineral that is a good heat and electrical conductor. With a melting point of roughly 3650°C, it is stable across a wide temperature range. Natural graphite comes in three varieties: High crystalline content, Amorphous, and Flake.
- Synthetic Graphite
Coke and pith are used to make synthetic graphite. In comparison to the natural one, it is less crystalline. Synthetic graphites are divided into two categories. The first is electro graphite, which is pure carbon created in an electric furnace from coal tar pitch and calcined petroleum coke. The second is synthetic graphite, which is made by heating calcined petroleum pitch to a temperature of 28000°C.
Uses of Graphite
[Click Here for Sample Questions]
- The mineral graphite is used to make pencil lead.
- Because of its capacity to absorb fast-moving neutrons, graphite is employed in nuclear reactors to regulate the nuclear fission reaction.
- Graphite is utilised as a lubricant in machine components due to its slippery nature.
- Because of its free electrons, graphite is employed as heat and electrical conductors in a variety of operations.
- Graphite is employed in high-temperature applications such as phosphorus and calcium carbide manufacturing.
- In aqueous electrolytic processes, such as the synthesis of halogens, graphite is utilised as an anode.
- As a carbon brush, graphite is employed as an electrical substance in electric motors.
- Crucibles are made from it because of its chemical resistance and high melting temperatures.
- For lithium-ion batteries, graphite materials are employed as the anode material.
Process of Making Graphite in a Lab
[Click Here for Sample Questions]
Graphite is a brittle, black-coloured steel composite utilised in a variety of industrial inductive operations, as well as in the lady for your pencil. We need the flowing material to produce graphite in the lab.
- Ten to fifteen grammes of sucrose
- Sulphuric acid, 18 molars, 10 mils
- Sodium bicarbonate (ten to twenty grammes)
- Beaker with a capacity of 250 milliliters
- A rod for stirring
- A graduated cylinder with a 10 ml capacity and a plastic zip-lock bag
To begin, fill a 10 millilitre graduated cylinder with 10 millilitres of 18 molar sulphuric acids. In a 50-millilitre beaker, put 15 grammes of sucrose. Pour the 10 mils of Cassatt punch chisel into the sucrose. Stir it vigorously. As the materials darken and finally turn black, you'll observe the reaction going place. The reaction is completed after some time, and the outcome is graphite.
Applications of Graphite
[Click Here for Sample Questions]
- Chemical Manufacturing
Graphite is employed in many hot areas in the chemical industry, such as in the manufacturing of phosphorus and calcium carbide in arc furnaces. In some electrolytic processes in liquids, such as halogen synthesis, graphite is utilised as an anode (chlorine and fluorine).
- The Nuclear Power Industry
In nuclear reactors, large amounts of high-purity electro graphite are utilised to make presidential sticks and display components. The lowest neutron absorption results in the formation of electro graphite with high thermal conductivity, high strength, and high temperatures.
- Applications of Electricity
Carbon brushes in electric motors include a significant quantity of graphite, which is employed as an electrical substance. The distance and structure have a big impact on a component's service life and performance.
- Applications in Mechanical Engineering
Piston rings, thrust bearings, journal bearings, and vanes are all examples of when graphite is employed as an engineering material. Carbon-based seals can be found in gasoline pumps and on the engine walls of a variety of aviation engines.
Also read: Valency of carbon
General Facts About Graphite
[Click Here for Sample Questions]
- Allotropy, also known as allotropism, is the property of some chemical elements to exist in two or more distinct forms, referred to as allotropes. Different structural changes of an element are known as allotropes. Carbon allotropes include graphite, diamond, and Florence.
- Carbon is a natural greenhouse gas, but there has been far too much carbon dioxide in the atmosphere since the industrial revolution. Humans have now become a non-natural impact on climate regulation. We consume far too many fossil fuels for transportation. Another human-caused phenomenon is deforestation.
- A covalent bond is a bond established by the sharing of electrons between two components.
Things to Remember
- The two most well-known variants of this carbon crystal, beta and alpha, have nearly identical material characteristics, with the exception that the layers of graphene stack differently. The alpha crystal might seem curved or flat.
- It is possible to mechanically convert one form to another, for example, from alpha to beta. The crystal would next be heated over 1300 °C to convert from beta to alpha.
- The carbon crystal has significantly anisotropic thermal and acoustic properties because photons travel rapidly across tightly constructed planes but move slowly from one plane to the next.
- The crystal is employed in a variety of applications, including electrodes and refractories for high-temperature processing, all for the simple reason that it is transparent. Graphite has excellent thermal and electrical conductivity, as well as excellent thermal stability.
Sample Questions
Ques. What is graphite in chemistry? (2 marks)
Ans - Graphite, commonly known as plumbago or black lead, is a carbon-based mineral. The layered structure of graphite is made up of rings of six carbon atoms organised in horizontal sheets that are widely separated.
Ques. What is the structure of graphite? (2 marks)
Ans - The structure of this crystal carbon is flat and layered. A graphene layer is a name given to each layer of graphene. Carbon atoms are organised in a honeycomb-like network in each layer, with a division of 0.142 nm and a plane spacing of 0.335 nm.
Ques. What are graphite and its uses? (2 marks)
Ans - Graphite is used in pencils and lubricants. It has a high heat and energy conductivity. Its high conductivity makes it helpful in electrical equipment including electrodes, batteries, and solar panels.
Ques. What is graphite made of? (2 marks)
Ans - Graphite is made up of a ring of six carbon atoms that are hexagonally linked together in widely separated layers. The bonds between the layers are strong, but the bonds between the layers are fewer and hence weaker.
Ques. What is the graphite formula? (2 marks)
Ans - Graphite's chemical formula is C, and its molecular weight is 12.01. Carbon allotropes include nanotubes, diamond, and graphite, and the chemical formula for carbon is "C"
Ques. What are the properties of graphite? (2 marks)
Ans - Graphite is a unique substance since it has both metal and non-metal qualities. Graphite is pliable but not elastic, and it has a high electrical and thermal conductivity. Chemically, it is inert and very refractory.
Ques. What is graphite colour? (2 marks)
Ans - Graphite is a grey tint made by combining blue, white, and black. Graphite and other neutral colours do not show on a typical colour wheel; instead, they exist alongside other 'non-colours' such as black and white.
Ques. Where is graphite found? (2 marks)
Ans - China has the world's greatest reserves, trailed by Mexico, the Czech Republic, Madagascar, and India. Graphite is produced in a number of different nations, including Canada, Germany, Sri Lanka, and North Korea. Open-pit and underground mining operations provide natural graphite.
Also Read:
Also Read:
| Guide Links | ||
|---|---|---|
| Basic Chemistry | Chemistry Formulas | Class 10 Science Notes |





Comments