Jasmine Grover Study Abroad Expert
Study Abroad Expert
An atom is the fundamental unit of matter that cannot be divided further into smaller parts. An ion, on the other hand, is formed due to the inequality of electrons and protons in an atom. Atoms consist of electrons, protons, and neutrons. An ion is created when an atom is electrically charged. The number of protons and electrons in an ion are unequal and therefore the ions are unstable in nature.
Key Terms: Atom, Ion, Protons, Electrons, Difference between atom and ion, nucleus
What is an Atom?
[Click Here for Sample Questions]
Atom is the smallest unit of matter that can’t be broken down. It can take part in different chemical reactions. A molecule is formed by a combination of atoms.
- The number of electrons and protons is equal in an atom.
- The nucleus of an atom is formed by neutrons and protons.
- The nucleus is surrounded by electrons.
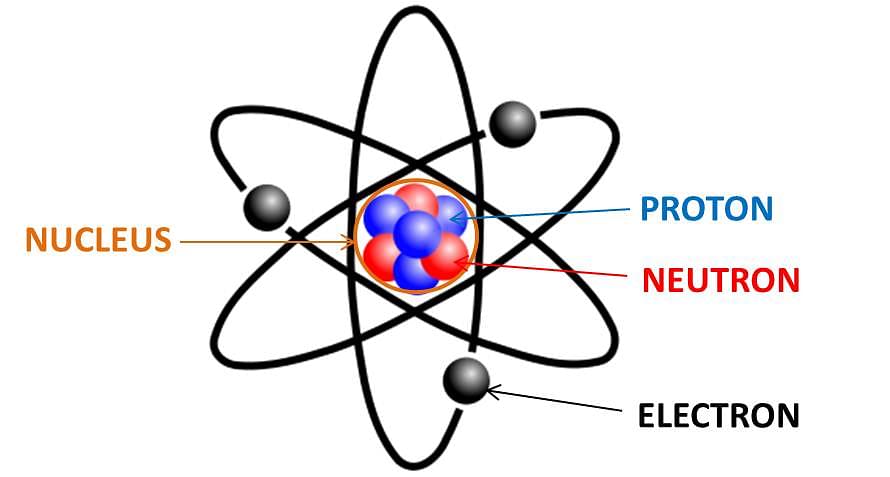
Atoms
Read More:
What is an Ion?
[Click Here for Sample Questions]
Ions are atoms with an unequal amount of protons and electrons. They are charged particles.
- Atoms are either positively charged or negatively charged.
- An atom with a maximum number of electrons is known as an anion.
- An atom with a maximum number of protons is known as a cation.
- Therefore, an ion is formed when an atom either gains or loses electrons.

Difference between Atom and Ion
[Click Here for Sample Questions]
The main difference between atom and ion are as tabulated below -
| Atom | Ion |
|---|---|
| An atom is the smallest unit of matter. | An ion exists in the form of either a single particle or a group of particles. |
| Atoms cannot exist independently in a solution. | Ions can independently exist in a solution. |
| A combination of atoms forms molecules | Ions form electrovalent bond |
| In the case of atoms, the number of electrons and protons is the same, therefore they are stable. | Electrons and protons are unequal in ions and hence they are unstable. |

Difference between atoms and ions
Properties of Atom
[Click Here for Sample Questions]
Examples of atoms include H, O, Fe, and N. Some of the properties of atoms include -
- Nuclear properties: Two atoms with the same proton number are similar chemical elements. Atoms having the same proton number but different neutron numbers are known as isotopes.
- Shape and size: Atoms don’t have a well-designed boundary, therefore their dimensions are defined by atomic radius. The smallest atom is helium with a 32 pm radius.
- Mass: The maximum mass of each atom is a combination of protons as well as neutrons. The total number of protons and neutrons present is known as the mass number. The mass number is dimensionless and is always a positive whole integer. The unit to express the mass of an atom is daltons (Da).
Read More: Writing Chemical Formulae
Properties of Ion
[Click Here for Sample Questions]
In the gaseous state, ions are highly reactive. When salts and solvents react with each other, ions are released in a solid as well as a liquid state. An ion can be distinguished as -
- Anion - The number of electrons in an anion is more than the number of protons. It’s denoted by the negative sign.
- Cation - The number of electrons in a cation is less than the protons. It’s denoted by a positive sign.
Atoms are electrically neutral whereas ions are charged particles. The combination of atoms results in molecules while ions combine to form compounds. Atoms can’t exist independently while ions can. Atoms can form an ionic or a covalent bond depending on the valency but ions can only form an ionic bond. Atoms take part in chemical reactions whereas ions take part only in ionic reactions. Ions are stable as they can have a complete octet. Atoms do not have a complete octet, hence they are unstable.
Read More: Difference Between Molar mass and Molecular mass
Things to Remember
- An atom is a basic unit of matter that is indivisible while an ion is formed when an atom gains or loses electrons.
- An atom that gains electrons is an anion.
- An atom that loses electrons is a cation.
- Atoms are stable while ions are not.
- Atmos form an ionic bond or a covalent bond whereas ions can only form ionic bonds.
Sample Questions
Ques 1: Which standard element is used for the atomic mass scale? (2 marks)
Ans: Carbon is used as a standard element for the atomic mass scale. The carbon atom consists of 6 neutrons and 6 protons in the nucleus and hence its mass number is 12.
Ques 2: How ions are formed? (2 marks)
Ans: Atoms achieve stability by acquiring the noble gas configuration, from which they lose or gain electrons, in order to attain the stable electronic configuration. As a consequence, ions are produced.
Ques 3: Define atoms. (1 mark)
Ans: The smallest parts of an element that keep its identity are called atoms.
Ques 4: Why do covalent compounds have lower melting points than ionic compounds? (3 marks)
Ans: Since the intermolecular connections that bind the molecules together in a molecular solid are weaker than the electrostatic attractions that bind oppositely charged ions together in an ionic solid, covalent compounds melt at lower temperatures than ionic compounds do.
Ques 5: In magnesium oxide molecules, name the cation and the anion present. (2 marks)
Ans: Magnesium oxide is written as MgO and in this molecule, the cation is Mg2+ and anion is O2- ions.
Ques 6: In 128g of Sulphur, calculate the number of Sulphur molecules present. (5 marks)
Ans: The gram molecular mass of sulphur (S8) = 32 X 8= 256 g
256g of Sulphur represents = 1 mol
128 g of Sulphur = (128 g X 1 mol)/256 g = 0.5 mol
The number of molecules present in S8 = 6.022 X 1023
0.5 mole of S8 has molecules = 0.5 mol/1.0 mol X 6.022 X 1023
= 3.011 X 1023
Ques 7: In 120 g of Nitric acid, calculate the number of oxygen atoms present. (3 marks)
Ans: The Molecular formula of Nitric Acid = HNO3
Gram Molecular mass of Nitric Acid = 1 + 14 + (3*16) = 63g
63g of HNO3 contains oxygen atoms = 3 X NO
Oxygen atoms present in 120 g of HNO3 = ((3 X NO) X 120g) / 63g= 5.714 X NO
= 5.714 X 6.022 X 1023
= 3.44 X 1024 atoms
Ques 8: Mention the chemical name of the following compounds: (3 marks)
i) ZnS
ii)AgBr
iii) K2SO4
iv) NH4Cl
v) Mg3(PO4)2
vi) Na3N
Ans: The chemical names of the above-mentioned compounds are as follows:
i) Zinc Sulphide
ii) Silver Bromide
iii) Potassium Sulphate
iv) Ammonium Chloride
v) Magnesium Phosphate
vi) Sodium Nitride
Do Check Out:




Comments