Muskan Shafi Education Content Expert
Education Content Expert
First 20 Elements of the Periodic Table give a great overview of the various element groups. The first 20 elements are found in more common chemical processes. Lithium, Beryllium, Sodium, Magnesium, Aluminium, Potassium, and Calcium are the metals found in the first twenty elements. Hydrogen, Helium, Carbon, Nitrogen, Oxygen, Fluorine, Neon, Phosphorous, Sulphur, Chlorine, and Argon are the non-metals found in the first twenty elements.
Read More: Periodic Classification of Elements
Key Terms: First 20 Elements, Periodic Table, Metals, Non-metals, Elements, Atomic Number, Atomic Mass
What are Elements?
[Click Here for Sample Questions]
An element is defined as a pure substance that consists of only one type of atom. Elements cannot be broken into two or more simpler substances by physical or chemical means.
- There are a total of 118 elements that have been discovered so far.
- Of the total elements, the first 94 are naturally occurring while the remaining 24 are man-made elements.
- All the elements are represented by their specific symbols assigned by IUPAC. For instance, Hydrogen is represented by H, Oxygen is represented by O, etc.
- The number of protons in the atomic nucleus of each element is used to determine its identity.
- By adding additional protons to an atom, a new element can be produced.
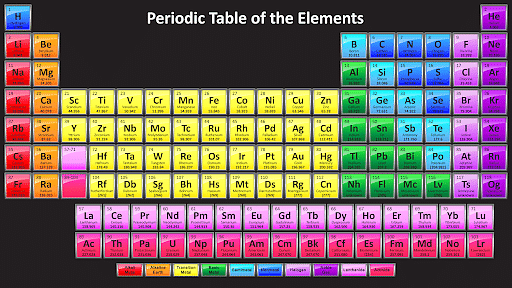
Modern Periodic Table
Read More:
First 20 Elements of Periodic Table
[Click Here for Previous Years Questions]
Tabulated below are the first 20 elements of the periodic table along with their atomic numbers and symbols.
| Element | Atomic Number | Symbol |
|---|---|---|
| Hydrogen | 1 | H |
| Helium | 2 | He |
| Lithium | 3 | Li |
| Beryllium | 4 | Be |
| Boron | 5 | B |
| Carbon | 6 | C |
| Nitrogen | 7 | N |
| Oxygen | 8 | O |
| Fluorine | 9 | F |
| Neon | 10 | Ne |
| Sodium | 11 | Na |
| Magnesium | 12 | Mg |
| Aluminum | 13 | Al |
| Silicon | 14 | Si |
| Phosphorous | 15 | P |
| Sulfur | 16 | S |
| Chlorine | 17 | Cl |
| Argon | 18 | Ar |
| Potassium | 19 | K |
| Calcium | 20 | Ca |
Mnemonic to Remember First 20 Elements
[Click Here for Sample Questions]
By giving memory aids for each word, the Mnemonic words make learning and recalling words and their meanings much easier. Here is the mnemonic for the first 20 elements of the periodic table:
| Element Name | Symbols | Mnemonic |
|---|---|---|
| Hydrogen | H | Hi |
| Helium | Hi | Hello |
| Lithium | Li | Listen |
| Beryllium | Be | B |
| Boron | B | B |
| Carbon | C | C |
| Nitrogen | N | News |
| Oxygen | O | O |
| Fluorine | F | F |
| Neon | Ne | New Zealand |
| Sodium | Na | Nagaland |
| Magnesium | Mg | Meghalaya |
| Aluminum | Al | All |
| Silicon | Si | Senior |
| Phosphorous | P | Public |
| Sulfur | S | Schools |
| Chlorine | Cl | Closed |
| Argon | Ar | Around |
| Potassium | K | Kargil |
| Calcium | Ca | Ca |
Importance of First 20 Elements of The Periodic Table
[Click Here for Previous Years Questions]
The first 20 elements of the periodic table are considered to be the most essential elements and have profound use in daily life. Given below is the importance of some of the first 20 elements:
- Oxygen (O): We all know that Oxygen plays a crucial role in respiration and we need oxygen for breathing. Oxygen is released through the process of photosynthesis in plants.
- Carbon (C): 18% of the human body comprises Carbon. All the essential compounds such as protein, sugar, and glucose contain carbon. Carbon is also present in fossil fuels such as petroleum and CNG.
- Aluminium (Al): Aluminium is widely used in our daily life as it is malleable and soft, and thus used in the making of aeroplane parts, utensils, etc.
- Calcium (Ca): Calcium is a very essential component that helps in maintaining bone strength.
- Silicon (Si): Silicon acts as a semiconductor and is used in computer chips.
- Phosphorus (P): Phosphorus is an essential component of ATP, which is the energy currency of the body. White Phosphorous is also used in the military to make weapons.
Also Read:
Noble Gases in First 20 Elements
[Click Here for Sample Questions]
Noble gases, also known as inert gases, are the least reactive or extremely non-reactive elements. The noble gases were discovered later than other elements.
There are three noble gases in the first 20 elements namely,
- Helium (He) with Atomic Number 2
- Neon (Ne) with Atomic Number 10
- Argon (Ar) with Atomic Number 18
Important Terms Related to Elements
[Click Here for Previous Years Questions]
- Atomic Mass: Every particle of matter, no matter how small or big, has some mass connected with it. The atomic mass is the mass of an atomic particle. This is often stated in terms of a unified atomic mass unit (amu) as per the international agreement.
- Atomic Number: The number of protons in an atom's nucleus or the number of electrons in an electrically neutral atom equals the atomic number. The number of protons in an atom is called its atomic number.
- Protons: They are subatomic particles that have a positive charge residing in the nucleus of an atom of the element.
- Neutrons: They are subatomic particles with a neutral charge. Neutrons and protons together add up to form the nucleus of the atom.
- Electrons: They are subatomic particles that are negatively charged. They usually orbit around the nucleus.
Read More: The d and f-Block Elements
Things to Remember
- An element is a substance that cannot be broken down or transformed into another substance by chemical methods.
- There are 118 known elements at present with the first 94 elements being natural and the rest 24 being man-made elements.
- The first 20 elements of the periodic table are very crucial and are an integral part of our daily lives.
- The symbols of elements are derived from the first letter of their names, with the second letter of their names occasionally added.
- The atomic number of an element refers to the order in which they appear on the periodic table.
Previous Years’ Questions (PYQs)
- The IUPAC symbol for the element with atomic number… (JEE Main 2019)
- Which of the following pairs has both members from the same…
- All elements in IIIA group, have…
- Which of the following has highest ionisation energy… (DUET 2008)
- Identify the wrong statement in the following… (NEET 2012)
- In the periodic table from left to right in a period, the… (NEET 1993)
- Correct order of 1st ionisation potential (IP) among following elements… (NEET 2001)
- The value of electronegativity of /atoms A and… (DUET 2009)
- Which one of the following ions has the highest value… (DUET 2008)
Sample Questions
Ques. What are elements? Name an element. (2 Marks)
Ans. An element is a substance that cannot be broken down or transformed into another substance by chemical methods. Hydrogen is an example of an element.
Ques. What is an atomic number? (2 Marks)
Ans. The number of protons in an atom's nucleus or the number of electrons in an electrically neutral atom is termed the atomic number.
Ques. What do you mean by the atomic mass of elements? (3 Marks)
Ans. The atomic mass of an element refers to the total mass of one atom of a specific element. Atomic Mass is equivalent to the number of protons and neutrons in the atom. Atomic Mass is expressed in terms of a unified atomic mass unit (amu).
Ques. What are the uses of Lithium, Phosphorous, Magnesium, Neon, and Argon elements of the periodic table? (5 Marks)
Ans. The uses of these elements of the periodic table are as follows-
- Lithium- Lithium is best recognized for its application in batteries. It's also employed in aluminium alloys to make cookware more robust, and, most strangely, as a mood stabilizer in psychiatric medications.
- Neon- The element neon is the fourth most plentiful in the universe. Advertising signs are by far the most common application of the element nowadays. The glass usually lights when tempted with electricity, leading to its use in the sign sector as well as high-voltage indicators and lasers. Neon is widely used in cryogenic refrigerant liquid.
- Magnesium- Epsom salts, milk of magnesia, chloride, and citrate are all examples of magnesium's medical use. Magnesium is also required for the survival of both animals and plants. It is frequently alloyed with aluminium for usage in-plane and automotive constructions due to its lower density than aluminium. It's also used to eliminate sulfur from molten iron and steel.
- Phosphorous- Flares and incendiary devices contain white phosphorus, while matchboxes contain red phosphorus in the substance attached to the side. Phosphorus compounds, on the other hand, are most commonly used in fertilizers. Phosphorus is also used in the manufacture of steel.
- Argon- Argon is utilized as a protective layer surrounding the filament in incandescent and fluorescent bulbs to prevent oxygen from corroding it. Arc welding and semiconductor crystals both employ it as a protective shield.
Ques. Name the noble gases present in the first 20 elements of the periodic table. (3 Marks)
Ans. There are mainly three noble gases in the first 20 elements of the periodic table:
- Helium (He)
- Neon (Ne)
- Argon (Ar)
The atomic numbers of Helium, Neon and Argon are 2, 10 and 18 respectively.
Ques. What are the chemical formulas of the first 20 elements? (5 Marks)
Ans. Diatomic elements include hydrogen, nitrogen, oxygen, fluorine, and chlorine. H2,N2,O2,F2,Cl2 are their chemical formulas, respectively.
The following elements have their symbol and chemical formula as:
- Helium-H,
- Lithium-Li,
- Beryllium-Be,
- Boron-B,
- Carbon-C,
- Neon-Ne,
- Sodium-Na,
- Magnesium-Mg,
- Aluminium-Al,
- Silicon-Si,
- Argon-Ar,
- Calcium-Ca
- Sulphur has the chemical formula S8, whereas phosphorus has the chemical formula P4.
Ques. What all information can we get from the atomic number of an element? (3 Marks)
Ans. The atomic number of an element provides the following information about the element:
- The atomic number tells us about the number of protons in the nucleus of an atom
- The number of electrons that surrounds the nucleus in a neutral atom.
Ques. Differentiate between an element and a compound. (3 Marks)
Ans. Elements are the pre substances that consist of one kind of atom only. Elements cannot be broken down and can exist as atoms or as molecules. Hydrogen and Oxygen are examples of elements.
Compounds, on the other hand, are substances consisting of two or more different types of elements in a fixed ratio of their atoms. Water (H2O) is an example of a compound.
Ques. What are the first 20 elements' valency and valence electrons? (5 Marks)
Ans. The first 20 elements' valency and valence electrons are:
| Elements | Valency | Valence Electron |
|---|---|---|
| Hydrogen | 1 | 1 |
| Helium | 0 | 2 |
| Lithium | 1 | 1 |
| Beryllium | 2 | 2 |
| Boron | 3 | 3 |
| Carbon | 4 | 4 |
| Nitrogen | 3 | 5 |
| Oxygen | 2 | 6 |
| Fluorine | 1 | 7 |
| Neon | 0 | 8 |
| Sodium | 1 | 1 |
| Magnesium | 2 | 2 |
| Aluminum | 3 | 3 |
| Silicon | 4 | 4 |
| Phosphorous | 3 and 5 | 5 |
| Sulfur | 2 | 6 |
| Chlorine | 1 | 7 |
| Argon | 0 | 8 |
| Calcium | 2 | 2 |




Comments