
Content Strategy Manager
Atoms are the fundamental building blocks of matter and the elements' defining structure. Because atoms were formerly supposed to be the tiniest things in the world and could not be separated, the name "atom" was derived from the Greek word "indivisible." Atoms are now known to be made up of three particles: protons, neutrons, and electrons, which are made up of even smaller particles called quarks. The electrons of an atom are drawn to its protons in the atomic nucleus by the electromagnetic force while the neutrons and protons are attracted to each other by a nuclear force. This force is usually greater than the electromagnetic force that repels positively charged protons from one another. After the Big Bang 13.7 billion years ago, atoms were formed. Conditions for the formation of quarks and electrons improved when the hot, dense new cosmos cooled. Quarks united to make protons and neutrons, which then merged to form nuclei. In this article, we will look at atoms and understand how they exist in nature.
Read More: Atoms and Molecules
| Table of Contents |
Keyterms: Atom, Electron, Proton, Neutron, Nucleus, Electromagnetic force, Cyclotron, Linear accelerator
What is an Atom?
Many ancient cultures, such as Greece and India, have the underlying belief that matter is made up of small, indivisible particles. The term atom comes from the Greek word atomos, which means "uncuttable." Although it has now been established that "atoms" may be separated.
- The proton has a positive charge. The atomic number of a chemical element is the number of protons in its nucleus. The rest mass of a proton, abbreviated mp, is roughly 1.673 x 10-27 kilograms (kg).
- A neutron is electrically neutral and has a rest mass of around 1.675 x 10-27 kg. When a proton or neutron travels at a high speed, such as in a cyclotron or linear accelerator, its mass increases.

Atom
Atomic Particles
The cosmos needed 3,80,000 years to cool down enough for the electrons to slow down enough for the nuclei to grab them and form the first atoms. According to reports, the first atoms were mostly hydrogen and helium, which are still the most plentiful elements in the universe. When the star erupted, gravity forced clouds of gas to congregate and form stars, and heavier atoms were (and still are) formed within the stars and transported across the cosmos (supernova).
The nucleus, which is at the center of the atom, contains heavier protons and neutrons than electrons.
- Electrons are extremely light and live in a cloud around the nucleus.
- Protons and neutrons have about the same mass.
In an atom, the number of protons and electrons is always the same, and the number of protons and neutrons is also usually the same. When a proton is added to an atom, it forms a new element; however, when a neutron is added to the same atom, it becomes an isotope or a heavier form of that atom.

Sub-atomic particles
Nucleus
Ernest Rutherford, a New Zealand scientist, discovered the nucleus in 1911.
- The positively charged particles of the atom were given the name proton by Rutherford in 1920.
- He also proposed the existence of a neutral particle within the nucleus, which was confirmed in 1932 by James Chadwick, a British physicist, and Rutherford's pupil.
- One of nature's four fundamental forces holds the nucleus together.
- According to the principles of electricity, this force between protons and neutrons overcomes the repulsive electrical force that would otherwise push the protons away.
- Because the binding force varies depending on the size of the nucleus, some atomic nuclei are unstable. These atoms will subsequently decay into other elements, such as carbon-14 degrading into nitrogen-14 in the case of carbon-14.

Atomic Nucleus
Read More:
Protons
Within atomic nuclei, protons are positively charged particles.
- Between 1911 and 1919, Rutherford conducted research with cathode-ray tubes and found the protons.
- Protons have a mass of 99.86 percent of neutrons.
- The chemical behavior of an element is determined by its number of protons.
- Each proton is formed of three quarks which include two up quarks and one down quark.

Protons
Electrons
Electrons are minuscule in comparison to protons and neutrons, being almost 1,800 times smaller than either.
- A British scientist named Joseph John (J.J.) Thomson discovered the electron in 1897.
- Electrons, which were once referred to as "corpuscles," contain a negative charge and are electrically attracted to positively charged protons.
- In the 1920s, Erwin Schrödinger, an Austrian scientist, proposed that electrons circle the atomic nucleus in routes called orbitals.
- The quantum model, often known as the electron cloud model, is the name given to this paradigm today.
- The electron configuration of an atom is the arrangement of electrons in a normal atom.
- Chemists can forecast an atom's characteristics, such as stability, boiling point, and conductivity, using electron configuration and physics principles.

Electrons
Read Also:- Classification of Organic Compounds
Neutrons
Rutherford proposed the existence of the neutron in 1920 and Chadwick found it in 1932. When atoms were fired at a thin layer of beryllium, the neutron, a subatomic particle with no charge, was unleashed.
- Neutrons are neutral particles that may be found in all atomic nuclei (except for hydrogen).
- The mass of a neutron is somewhat more than that of a proton.
- Neutrons are made up of quarks, just like protons, with one "up" quark (with a positive 2/3 charge) and two "down" quarks (each with a negative one-third charge).

Neutrons
History of an atom
Democritus, a Greek scientist, and philosopher, first proposed the hypothesis of the atom around 440 B.C. Democritus begins his explanation of the atom with a stone. When you cut a stone in half, you get two parts of the same stone. If the stone were repeatedly sliced, a chunk of the stone would eventually become too tiny to cut. The word "atom" is derived from the Greek word "indivisible," which Democritus determined to be the point at which a being (any sort of substance) can no longer be separated. Democritus' atomic theory was ignored, however, because most philosophers at the time thought that all matter was generated from the earth, air, fire, and water.
Thomson, the British scientist who discovered the electron in 1897, established that atoms may be separated. By investigating the features of electric discharge in cathode-ray tubes, he was able to prove the existence of electrons. The rays were deflected within the tube, according to Thomson's 1897 study, proving that there was something negatively charged within the vacuum tube. Thomson provided a description of his idea of the atom, dubbed the "plum pudding model," in 1899.
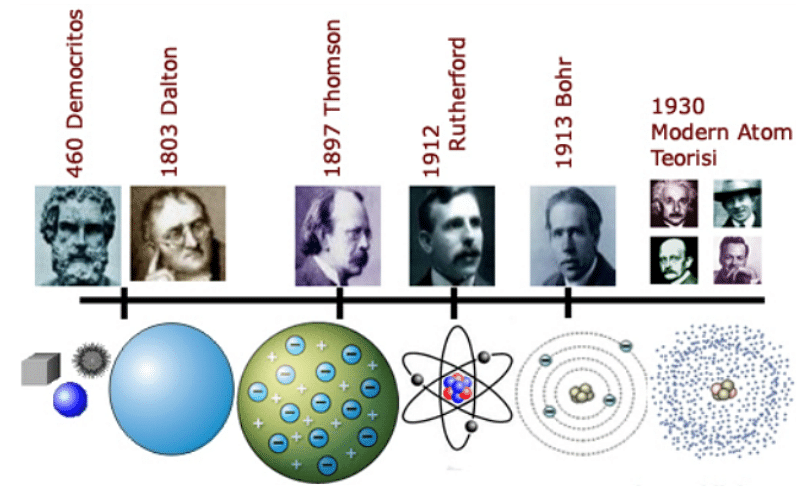
History of Atom
Niels Bohr (who built on Rutherford's model to include properties of electrons based on the hydrogen spectrum), Erwin Schrödinger (who developed the quantum model of the atom), Werner Heisenberg (who stated that one cannot know both the position and velocity of an electron at the same time), Murray Gell-Mann, and George Zweig all contributed to the atomic model (developed independently the notion that protons and neutrons are made out of quarks).
Also check:
How do atoms exist?
Ernest Rutherford headed a team that shot particles at atoms and carefully examined them, based on a popular set of experiments from the early twentieth century. It demonstrated how the orbits in the inside of a normal atom were structured. Furthermore, certain compounds are known to be radioactive, which means that they spontaneously break into simpler molecules and release small particles as a result.
The newly developed study of thermodynamics provided one indication of the existence of atoms. Physicists recognized that they could see gases and fluids as if they were made up of an infinite number of small, even microscopic particles in order to grasp how heat engines functioned, as well as all the associated notions like temperature, pressure, and entropy. For instance, "temperature" refers to the average speed of all the gas particles that collide with your thermometer and transmit energy to it.
Atoms can exist in two forms:
- In molecular form
Molecules can be formed up of the same element's atoms or atoms from distinct elements. Two Oxygen atoms, for example, unite to form O2.
- It takes the form of ions
Ions are generated when an atom loses or gains electrons. For example, common salt, also known as sodium chloride, is made up of millions of sodium ions (Na+) and chloride ions (Cl-).
Structure of Atom
Ernest Rutherford, a scientist, devised an early model of the atom in 1912. He was the first to propose that atoms are similar to small solar systems, with the exception that the attractive attraction is produced by opposing electrical charges rather than gravity. Electrons orbit the nucleus in a circular motion in the Rutherford atom.
In 1913, Niels Bohr amended Rutherford's hypothesis. The negatively charged electrons in the Bohr atom circle the nucleus at defined median distances. These distances are represented by spheres called shells that surround the nucleus. Electrons have the ability to hop from one shell to another. When an electron absorbs a certain amount of energy, it moves to a higher, shell. When it loses a certain amount of energy, it descends to a lower shell.

Atomic Structure
The atomic mass, or atomic weight, is the entire mass of an atom, including protons, neutrons, and electrons. Only a small portion of this mass is made up of electrons. Because the amount of neutrons in an atom varies, most elements have multiple distinct atomic weights.
Atoms with the same number of protons but varying numbers of neutrons belong to the same element but are referred to as various isotopes. The sum of the number of protons and neutrons determines the isotope of an element. Carbon 12 (the most prevalent, non-radioactive isotope of carbon) and carbon 14 are examples of distinct isotopes of an element (a less common, radioactive isotope of carbon).
Protons and electrons have the same charge and repel each other, and an atom generally contains an equal quantity of each. As a result, most atoms are neutral.
An anion is a negatively charged ion with additional electrons, whereas a cation is a positively charged ion with a lack of electrons.
Things to Remember
- Protons reside in a nucleus and have a positive nuclear charge.
- The atomic number, often known as the proton number, is the total number of protons in an atom. An element's atomic number identifies it (e.g., the element of atomic number 6 is carbon).
- Cathode rays are negatively charged basic particles and are known as electrons.
- Electrons are found in the electron cloud, which is the region around the atom's nucleus. The chances of locating an electron closer to the nucleus of an atom are generally higher.
- Electrons have a negative charge that is the same magnitude as protons' positive charge.
- Ions are made up of protons and electrons in unequal numbers, resulting in positive cations or negative anions.
- The neutron number is the number of neutrons obtained by subtracting the proton number from the atomic mass number.
- Beta particles are high-energy, high-speed free electrons or positrons that are released during the beta decay process.
Sample Questions
Ques. What are subatomic particles? (5 marks)
Ans. The particles that combine together to form an atom are known as subatomic particles. This includes protons, electrons, and neutrons in general.
- A strong force holds the nucleus together. According to the principles of electricity, this force between protons and neutrons overcomes the repulsive electrical force that would otherwise push the protons away.
- Within atomic nuclei, protons are positively charged particles. Between 1911 and 1919, Rutherford conducted research with cathode-ray tubes and found them. Protons have a mass of 99.86 percent of neutrons.
- The electron configuration of an atom is the arrangement of electrons in a normal atom. Chemists can forecast an atom's characteristics, such as stability, boiling point, and conductivity, using electron configuration.
Ques. What is the atom's tiniest component? (2 marks)
Ans. The smallest of the three particles that make up an atom is an electron. Electrons are located in orbitals or shells that encircle an atom's nucleus.
Ques. How do isotope’s atomic structures differ? (1 mark)
Ans. The atomic structure of isotopes differs in terms of the total amount of neutrons in the atom's nucleus, as shown by their nucleon numbers.
Ques. Is it possible to destroy atoms? (2 marks)
Ans. No atoms can be destroyed or formed. The final line is that matter exists in many distinct forms across the cosmos. Matter does not appear or disappear after any physical or chemical process. Every living and nonliving object on Earth—including you—is made up of atoms generated in the stars (a very, very long time ago).
Ques. What are some of the flaws in Bohr's atomic model? (3marks)
Ans. The flaws in Bohr’s atomic model are:
- The structure of an atom, according to this atomic model, provides poor spectrum predictions for bigger atoms.
- It also didn't account for the Zeeman effect. Only the hydrogen spectrum could be explained correctly.
Ques. What is the atom's fastest traveling particle? (2 marks)
Ans. Neutrinos are subatomic particles with nearly no mass that can whiz through planets as if they don't exist. Neutrinos should move at almost the speed of light, which is around 186,000 miles (299,338 kilometers) per second, due to their near masslessness.
Ques. Are neutrons found in all atoms? (3 marks)
Ans. Except for one, all elements contain neutron-rich atoms. In the nucleus of a typical hydrogen (H) atom, there are no neutrons. There is only one electron and one proton in that teeny-tiny atom (the smallest of all). Deuterium has one additional neutron, whereas tritium has two more neutrons.
Ques. Who is referred as the "Father of the Atomic Theory"? (2 marks)
Ans. John Dalton (1766-1844) pioneered the theory that everything is formed of atoms in a book published in 1808. He is frequently referred to be the "father" of atomic theory.
Also check:





Comments