Collegedunia Team Content Curator
Content Curator
Examples of bases include potassium oxide, sodium hydroxide, and calcium carbonate. Some of the other common examples of bases are ammonium hydroxide, metal oxides and metal hydroxides. A base can be defined as a substance which neutralizes acid by reacting with hydrogen ions.
- Most of the bases are known to be minerals which react with acids in order to form water and salts.
- Bases generally comprise oxides, hydroxides and carbonates of metals.
- The bases which are soluble are known as Alkalis. For instance, Sodium Hydroxide is an Alkali.
- However, since Copper (II) oxide is not soluble in water, it is considered a base, but not an Alkali.
- Thus, it can be said that all alkalis are bases, but not all bases are alkalis.
- Bases are typically bitter to taste and slippery to touch. They are known to be proton (H+) acceptors.
Read Also: Importance of pH in Everyday Life
| Table of Content |
Key Terms: Bases, Solvent, Detergents, Potassium Hydroxide, Sodium Hydroxide, Ammonium Hydroxide, Alcohol, Ionic Compounds
What are Bases?
[Click Here for Sample Questions]
Bases can be defined as:
| “The chemical substances which tend to donate electrons, liberate hydroxide ions (OH– ions), and when dissolved in water, accept protons (H+ ions)” |
- Bases are chemical substances that are bitter and slippery (in water).
- It reacts with acids to form salts and sometimes act as a catalyst to promote reactions.
- Bases usually have a pH level of greater than 7.
- A solution of aqueous base is often used in order to conduct electricity.
- Bases are ionic compounds that produce negative hydroxide ion (OH-) when reacted with water.
- An example is when Sodium chloride reacts with water, resulting in the production of negative hydroxide ion(OH-) and positive sodium ion (Na+).
The reaction is represented in the form of the equation below.
⇒ Na-OH+ (s) + H2O(l) OH-(aq) + Na+(aq)
Properties of BaseSome of the important properties of bases include:
|
Types of Bases
[Click Here for Sample Questions]
The types of bases include:
- Strong base: A strong base is any compound which can remove a proton from a weak acid or, when in water, dissociate into its ions. Some examples of strong bases include potassium hydroxide (KOH), and sodium hydroxide (NaOH).
- Weak base: Weak bases, when in water, have an incomplete dissociation. The aqueous solution comprises both the weak base and its conjugate acid. Some examples of the same are ammonia (NH3), water (H2O), and pyridine (C5H5N).
- Superbase: Superbases are those bases which are better at deprotonation as opposed to strong bases. Superbase has a very weak conjugate acid. However, it can be acquired by mixing an alkali metal with a conjugate acid. Some examples of the same are sodium hydride (NaH), and ortho-diethynylbenzene dianion (C6H4(C2)2)2−
- Neutral base: It is seen to form a bond with a neutral acid and share an electron pair.
- Solid base: Solid base can is active in solid form. Some examples of the same include silicon dioxide and sodium hydroxide placed on alumina.
Read more:
Examples of Bases
[Click Here for Sample Questions]
Out of the many base examples, these are some of them:
Potassium Hydroxide (KOH) (caustic potash)
Potassium hydroxide, also known by the name lye, is an inorganic compound which has the chemical formula KOH.
- Potassium Hydroxide has a molecular weight of 56.106.
- Potassium hydroxide is a white, odourless solid or clear aqueous solution.
- Boiling point of KOH Is 1327°C.
- Melting point of KOH is 360°C.
- The PH of KOH is 13.5.
- KOH is very dangerous and can even burn the skin, if it comes in direct contact.
- KOH is used in a variety of industries ranging from food, cleaning and pharma.
- It is also used in the glass industry and as an electrolyte in production of alkaline batteries.
- The potassium hydroxide is much softer than other hydroxides. This is the reason why bathing soaps and liquids uses potassium hydroxide rather than other hydroxides.

Read More: Acids, Bases and Salts
Sodium Hydroxide (NaOH)
Sodium hydroxide, which is also called caustic soda, is an inorganic compound that has the formula NaOH.

- Sodium Hydroxide has a molecular weight 39.97.
- NaOH is a pure white crystalline solid. It is odourless.
- Boiling point of NaOH is 1390°C.
- Melting point if NaOH is 318.4°C.
- The pH value of NaOH is 12.88.
- Contact with concentrated NaOH can be very dangerous as it can permanently damage many vital organs like eyes, lungs and skin. In some conditions, it may even lead to death.
- Sodium Hydroxide is a very widely used industrial product and is used in production of cleaning products like detergents. Also used in the paper industry, dying industry and petroleum industry.
- NaOH is a strong base, and when it reacts with any acid, it forms water and sodium salt.
One of the most famous reaction is when sodium hydroxide reacts with Hydrochloric Acid which results in the formation of water and sodium chloride (Table Salt). Thus,
- NaOH + HCl → H2O + NaCl
- NaOH + HX → H2O + NaX
Another very famous and important reaction related to sodium hydroxide is when sodium hydroxide reacts with Carbon Dioxide, this led to the creation of sodium carbonate and water. Thus,
- 2NaOH +CO2 → Na2CO3 + H2O
Sodium Hydroxide is harsher but very effective in cleaning. This is the reason why many detergents widely use sodium hydroxide instead of potassium hydroxide.
Barium Hydroxide (Ba(OH)2)
Barium hydroxide is white and odourless substance. Usually available in form of granular form.

- The Molecular Weight of Barium Hydroxide is 171.34.
- The boiling point of Ba(OH)2 is 780°C.
- Melting Point of Ba(OH) 2 is 78°C.
- PH of Ba(OH) 2 is 12.
- Ba(OH) 2 is harmful if swallowed or inhaled. If done so it can severely damage the internal organs like respiratory organs, eyes and skin.
- Barium Hydroxide is used in the manufacturing of alkalis and oil. It is also widely used in manufacturing of glass.
Cesium Hydroxide (CsOH)
Caesium hydroxide is kown to be a strong base, comprising the highly reactive alkali metal, Caesium
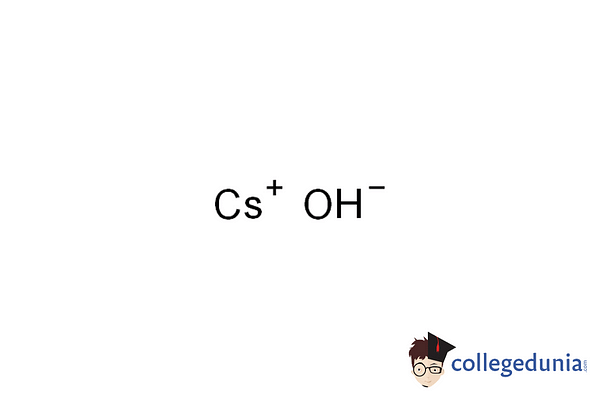
- Molecular Weight of Cesium Hydroxide is 149.913.
- CsOH is colourless to yellow in colour. Is a crystalline solid substance.
- Boiling point of CsOH is not known.
- Melting Point of CsOH is 272°C.
- Cesium Hydroxide is very corrosive and dangerous to the eyes and intestine. The chemical is very dangerous to human skin.
- Cesium Hydroxide is a very important raw material in the manufacturing of polyurethane Foams.
- It is also recommended to be used in alkaline storage batteries.
Calcium Hydroxide (Ca(OH)2)
Calcium Hydroxide has white powdery appearance or colourless crystalline appearance. Calcium hydroxide (also known as slaked lime) is an inorganic compound that has the chemical formula Ca(OH)2.

- The molecular weight of 74.093. Calcium hydroxide is commonly called as slaked lime.
- The boiling point of Calcium Hydroxide is 2850°C.
- The melting Point of Calcium Hydroxide is 580°C.
- The pH value of Ca(OH)2 is 12.67.
- Unprotected exposure to Ca(OH)2 can cause very severe skin irritation and burn on the skin. If by any chance the chemical is swallowed, it can also cause internal organ damage.
- Calcium hydroxide is very widely used. It is used in sewage treatment and food processing. It is also used in the construction sector. Also very widely used in the dental industry for root canal filling.
An important reaction to remember is related to the formation of carbon hydroxide. When carbon dioxide is reacted with water, it forms carbon hydroxide:
- CaCO3(s) → CaO + CO2(g) … (1)
- CaO(s) +H2O → Ca(OH)2(s) … (2)
- Ca(OH)2(s) + CO2(g) → CaCO3(s) + H2O … (3)
Zinc Hydroxide Zn(OH)2
Zinc hydroxide is known to be a weak base. It can be physically seen as a white powder with a chemical formula of Zn(OH)2.
- It occurs naturally, but this substance can also be formed in the lab.
- Zinc Hydroxide can be acquired by adding sodium hydroxide to a zinc salt solution.
- This substance has a molecular mass of 99.424 g/mol with a density of 3.053 g/cm³.
- It has a melting point of 125 °C.
Rubidium Hydroxide (RbOH)
Rubidium hydroxide is known to be a strong base. With a greyish-white color, this substance has a chemical formula RbOH.
- It is also known as rubidium hydrate.
- It is prepared in a lab as it does not occur naturally.
- The molecular mass of Rubidium Hydroxide is of 102.475 g/mol and has a density of 3.2 g/cm³.
- The boiling point of Rubidium Hydroxide is 1,390 °C, while its melting point is 301 °C.
- It is very corrosive in nature.
Also Read:
Things to Remember
- Bases are chemical substances that donate electrons, release hydroxide ions (OH– ions), and when dissolved in water, they accept protons (H+ ions).
- Many strong bases like sodium hydroxide and potassium hydroxide have very high pH (pH >12).
- Whenever a base reacts with water, it dissociates its ions. The extent of dissociation varies with the strength of the base.
- Strong bases dissociate completely when they react with water.
- When a strong base reacts with strong acid, it will always result in the formation of water and salt.
- Ionic metal oxides react with water, forming a hydroxide and making the solution basic in nature.
- Strong basic hydroxide reacting with water, leads to the formation of carbonate.
Sample Questions
Ques: A white-coloured powder is prevalent for supporting fractured bones. What is the chemical name and formula of that powder? (1 Mark)
Ans: The white-coloured powder which is prevalent for supporting fractured bones is Calcium Sulphate Hemihydrate. The formula of Calcium Sulphate Hemihydrate CaSO4. ½ H2O.
Ques: Give a reason why sulfuric acid is a strong acid and acetic acid a weak acid? (1 Mark)
Ans: Sulfuric acid is considered a strong acid since it dissociates into ions completely. In that process, it liberates H+ in an aqueous solution. However, acetic acid is considered a weak acid only since it does not dissociate in an aqueous solution entirely.
Ques: Which among the given list is the strongest acid?
a) CH2ClCOOH
b) CH3COOH
c) CHCl2COOH
d) CCl3COOH (1 Mark)
Ans: (d) CCl3COOH
Explanation: Strong acid implies that it has weak conjugate base. Thus, CCl3COO– < CHCl2COO– < CH2ClCOO– < CH3COO–. Hence, the strongest acid in this case is CCl3COOH.
Ques: State Reasons:
(i) Tap water is known to conduct electricity, while distilled water is known not to.
(ii) Dry hydrogen chloride gas doesn’t turn blue litmus red. However, dilute hydrochloric acid does. (2 Marks)
Ans: (i) Tap water comprises ions that help to conduct electricity. Distilled water, however, does not comprise ions.
(ii) Dry HCl is known not to form ions but HCl gives H+ and Cl–.
Ques: Briefly explain what are Acids, Bases and Salts? (3 Marks)
Ans: Acid: A chemical compound that gives a sour taste and has a pH value of less than 7. Examples include citric acid found in likes of lemon juice, lactic acid found in milk and milk products.
Base: A chemical compound that gives a bitter taste and has a value more than 7 on a pH scale. For example, sodium hydroxide (NaOH), potassium hydroxide (KOH), etc.
Salt: A substance that possesses no odour, gives salty taste and readily dissolves in water. It has a pH value exactly equal to 7.
Ques: How do you determine the strength of a Base solution? (1 Mark)
Ans: The strength of base solution is determined by the amount of OH- ions produced. Generally, a universal indicator or a pH indicator is used to depict the basicity of the solution. If the solution contains a higher number of OH- ions, the pH reading moves between 7-14, 14 being the most alkaline.
Ques: Are all bases soluble in water? (1 Mark)
Ans: Not all bases are readily soluble in water, only few bases characterised under the term ‘Alkalis’ are soluble in water.
Ques: Explain the term ‘weak bases’ and give examples for the same. (2 Marks)
Ans: A weak base is a type of base which does not completely dissolve in water, may or may not form a residue or float on the water surface. Examples of weak bases include
- Ammonia (NH3)
- Copper Hydroxide (Cu(OH)2)
- Methylamine (CH3NH2) etc
Ques.: Briefly list the properties of Bases. (3 Marks)
Ans: Some of the properties of Bases include:
- Bases possess higher pH value i.e., above 7.
- Bases conduct electricity in solution form.
- Bases tend to release OH- ions in an aqueous solution.
- Bases turn the red litmus blue
- They have a soapy texture and are bitter in taste.
Ques: Define the meaning of water of crystallization of a substance. Represent an activity which shows that blue copper sulphate crystals possess water of crystallization. (4 Marks)
Ans. The fixed number of water molecules that can be found in one unit formula of salt in its crystalline form is known as the water of crystallisation.
An example of the same is CuSO4.5H2O.
This means that one formula unit of copper sulphate is known to possess five water molecules.
Thus, an activity to demonstrate the crystallisation of water is:
1) In a dry test tube, place copper sulphate crystals and heat it.
2) The water droplets can be seen in the test tube walls, and the salt turns white.
3) Further, place about 2 – 3 drops of water on the heated copper sulphate sample.
4) It can be seen that the blue colour of copper sulphate crystals restores back.
This happens because:
1) As can be seen in the activity above, dry copper sulphate crystals typically contain water of crystallisation. However, when heated, the water in the crystals evaporates, turning the salt white.
2) The blue colour returns back when the crystals are further moistened with water.
Also Read:








Comments