Arpita Srivastava Content Writer
Content Writer
The key difference between baking soda and baking powder is that chemically, they are very different. However, they are both used as leavening agents, which makes it important to understand the difference between them.
- Baking powder is a combination of sodium bicarbonate, other bicarbonates, and acid salts.
- It is a leavening agent which is produced by a mixture of an acid and an alkali.
- Baking soda is sodium bicarbonate, which is denoted by the chemical formula NaHCO3.
- It is prepared by using Solvay’s process.
- Baking soda is used in the preparation of soft and fluffy bread and cakes.
- Baking powder is used for cleaning kitchen countertops.
Key Terms: Difference between Baking Soda and Baking Powder, Baking Soda, Baking Powder, Sodium Bicarbonate, Bicarbonates, Acid, Salts, Bases, Solvay’s Process, Sodium Aluminium Sulphate
What is Baking Powder?
[Click Here for Sample Questions]
Baking powders are a product of bases, acids, and some buffering materials. These materials help in preventing early acid-base reactions. Baking powder is an essential ingredient in many recipes since it helps in leavening and increasing volume.
- Baking powder works in the same way as leavening agents to improve the texture of baked products.
- It is used to increase the volume and lighten the texture of baked goods.
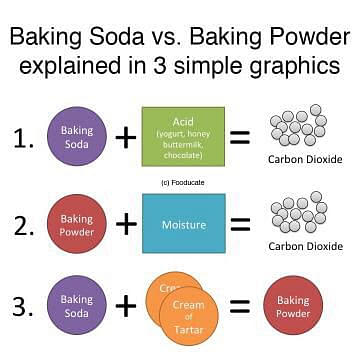
- The baking powder is a mixture of a carbonate or bicarbonate and a weak acid.
Food manufacturer Alfred Bird produced the first single-acting baking powder. Single-acting means that all carbon dioxide is released when it is dampened.
- An unopened packet of powder has a shell life of 6 months.
- It is sometimes known as double-acting.
Chemical Reaction of Baking Powder
The chemical reactions involved in the preparation of baking powder are as follows:
- In the chemical reaction of baking powder, an H+ ion is used.
- Na+ ions are released by sodium bicarbonate, as per the reaction below.
- Here, we have assumed that cream of tartar is used as the acidic component:
NaHCO3 + KHC4H4O6 → KNaC4H4O6 + H2O + CO2
If another acidic component is also added in baking powder (eg: sodium aluminium sulphate), the baking powder also gives a second rise to the dough or batter when heated.
- The reaction at high temperature is:
NaAl(SO4)2 + 3NaHCO3 → Al(OH)3 + 2Na2SO4 + 3CO2
Baking Soda and Baking Powder
Read more:
| Chapter Related Links | ||
|---|---|---|
| Lewis acid and base | Base Meaning | Hydroxide |
| Aluminum Hydroxide | Sulphur dioxide | Bases |
What is Baking Soda?
[Click Here for Sample Questions]
Baking soda is a salt that is composed of a sodium cation. It can be denoted as Na+, and a bicarbonate anion can be denoted as HCO3–.
- Baking soda is a white, crystalline solid with a salty taste.
- It is used as a leavening agent in baking.
- A leavening agent causes foaming, which leads to softening of the mixture.
Baking soda is a weak base and has a pH value of about 8.31. The chemical formula of the compound is Sodium Bicarbonate or Sodium Hydrogen Carbonate.
- It is a carbonic acid monosodium salt, which is composed of carbon, sodium, oxygen, and hydrogen.
- A French chemist named Nicolas Leblanc invented the baking soda.
- Naturally occurring sodium bicarbonate is found in the mineral nahcolite.

Chemical Reaction of Baking Soda
Chemical Reaction of Baking Soda
Baking soda reacts with acids and forms the carbon dioxide gas. The chemical reaction which takes place is:
NaHCO3 + H+ → Na+ + CO2 + H2O
- In order to process this reaction, several edible items can be used as acids (or providers of H+ ions).
- Some of the commonly used items are curd (lactic acid), vinegar (acetic acid), and citrus juice (citric acid).
- Sodium bicarbonate gets decomposed into sodium carbonate, water, and carbon dioxide gas when heated.
- This process is termed as thermal decomposition, which takes place at temperatures above 80°C.
2NaHCO3 → Na2CO3 + H2O + CO2
This is not a preferred method to use baking soda as a leavening agent because sodium carbonate, thus formed, can alter the taste of the end product. Baking soda is both mined and produced in factories.
- In factories, it is produced by reacting carbon dioxide and water with sodium carbonate.
Na2CO3 + CO2 + H2O → 2 NaHCO3

Baking Soda and Baking Powder
Difference between Baking Soda and Baking Powder
[Click Here for Sample Questions]
The main differences between Baking Soda and Baking Powder are listed below:
| Baking Soda | Baking Powder |
|---|---|
| Baking soda contains one ingredient – Sodium Bicarbonate | Baking powder consists of multiple ingredients including Bicarbonates and acid salts. |
| It does not contain Monocalcium Phosphate. | It contains Monocalcium Phosphate, which reacts with NaHCO3 when water is added or it is heated. |
| The compound reacts immediately with acids | The compound does not immediately react with acids. |
| It has a short leavening process | The leavening process extends with interference of a second acid. |
| Baking products produced with baking soda are not as fluffy when compared to baking powder products. | It gives fluffier texture to baked products. |
Also read:
Things to Remember
[Click Here for Sample Questions]
- The difference between baking soda and baking powder depends on the compositions and functions.
- Baking powder is a leavening agent that contains both sodium bicarbonate and an acidic ingredient.
- It releases carbon dioxide at a faster rate through an acid-base reaction than fermentation.
- The compound significantly reduces the time and labour required for the production of baking products.
- Baking soda (chemically known as sodium bicarbonate) is a baking ingredient that gets activated by a liquid and an acid.
- The compound doesn’t have any acidifying or drying agents.
- Baking soda has weak disinfectant properties and is used as a fungicide against some microorganisms.
Sample Questions
Ques. What is the effect of baking powder? (2 marks)
Ans. Baking powder is a two-in-one leavening compound that mixes a powdered alkali (sodium bicarbonate) with a powdered acid (originally tartaric acid). A chemical reaction that produces carbon dioxide gas, inflating cookies, cakes, and pancakes occurs when moistened in a dough or batter.
Ques. What is the purpose of baking powder? (2 marks)
Ans. Baking powder is used to increase the volume of baked goods and to lighten the texture. It works by releasing carbon dioxide gas through an acid-base reaction into a batter or dough, causing expansion of bubbles in the wet mixture, leavening the mixture.
Ques. How can baking soda be converted into baking powder? (2 marks)
(a) Adding nitric acid
(b) Adding hydrochloric acid
(c) Adding sulphuric acid
(d) Adding tartaric acid
Ans. Baking soda when mixed with acid, specifically tartaric acid converts baking soda into baking powder. Tartaric acid is to neutralise sodium carbonate and cake will not taste bitter. The real reactions are more complicated because the acids are complicated. For example starting baking soda and monocalcium phosphate is proposed to react to produce carbon dioxide by:
14NaHCO3 + 5Ca(H2PO4)2H2O → 14CO2 + Ca5(PO4)3OH + 7Na2HPO4 + 18H2O
Ques. Salt A commonly used in bakery products on heating gets converted into another salt B which itself is used for removal of hardness of water and a gas C is evolved. The gas C when passed through lime water, turns it milky. Identify A, B and C? (4 marks)
Ans. Baking powder which is a salt is used as a bakery product which gives sodium carbonate and carbon dioxide gas on heating. Sodium carbonate is used to remove the hardness of water. Carbon dioxide turns lime water milky.
2NaHCO3 + Heat ----→ Na2CO3 + CO2 + H2O
Therefore,
- Salt A is sodium bicarbonate and used as baking powder.
- Salt B is sodium carbonate, which is used to remove the hardness of water.
- The C is carbon dioxide gas which turns lime water milky.
Ques. How would you distinguish between baking powder and washing soda by heating? (4 marks)
Ans. The production of carbon dioxide is the main characteristic of baking soda that makes it suitable for baking. Baking soda gives carbon dioxide and water vapour on heating at even low temperatures such as 100°C. The gas so formed turns lime water milky, which confirms the presence of carbon dioxide gas.
2NaHCO3 + Heat → Na2CO3 + CO2 + H2O
When Washing soda (Na2CO3) is heated it does not produce carbon dioxide even at high temperatures, such as 200°C or 300°C. However sodium carbonate gives sodium oxide and carbon dioxide when heated at about 1000°C.
Na2CO3.10H2O + Heat → Na2CO3 + 10H2O
The gas produced by heating of the samples can be checked by passing through lime water. If the lime water turns milky then the evolved gas is carbon dioxide and the heated substance is baking powder.
Ques. Explain the production of baking soda (sodium bicarbonate)? (4 marks)
Ans. (a) By Solvay Process: Baking soda (Sodium bicarbonate) is produced by reaction of sodium chloride, carbon dioxide and ammonia. This process is known as Solvay Process.
NaCl (Sodium Chloride) + CO2 (Carbon dioxide) + NH3 (Ammonia) + H2O (Water) → NH4Cl (Ammonium Chloride) + NaHCO3 (Baking Soda)
(b) When carbon dioxide is passed through the solution of sodium hydroxide, sodium carbonate is produced. After passing more carbon dioxide, this sodium carbonate (baking soda) changes into sodium bicarbonate.
2NaOH (Sodium Hydroxide) + CO2 (Carbon dioxide) → Na2CO3 (Sodium Carbonate) + H2O (Water)
Na2CO3 (Sodium Carbonate) + CO2 (Carbon dioxide) → H2O (Water) + NaHCO3 (Baking Soda)
Ques. What are the properties of baking soda? (3 marks)
Ans. The properties of baking soda are as follows:
- Baking soda is a non-flammable compound.
- It has a melting point of 50 degree celsius.
- It is white crystalline solid which is odourless in smell.
- The compound is a weak base with a pH of 8.3.
Ques. What are the uses of baking soda? (3 marks)
Ans. The uses of baking soda are as follows:
- Baking soda is used to relieve heat burn.
- The compound is used in the preparation of cakes and bread.
- It is used for whitening of teeth.
- The compound is used as pesticide and used for removal of dirt.
- Baking soda is used in ear drops, cosmetics and personal care products.
Ques. What are the uses of baking powder? (2 marks)
Ans. The uses of baking powder are as follows:
- Baking powder is used in cleaning household items.
- It is used in baking of cakes, muffins, bread and other products.
Ques. How can we substitute baking powder with baking soda? (3 marks)
Ans. Baking powder can be substituted by baking soda by adding an acidic ingredient to the powder, which will provide the necessary carbon dioxide release for leavening. The general rule of thumb that is used for substituting baking powder involves the addition of ¼ teaspoon of baking soda to every teaspoon of baking powder added. However, the correct amount of addition may vary depending on the recipe and the acidic ingredients present.
Ques. What will happen if baking soda and baking powder are mixed together? (2 marks)
Ans. If you combine baking powder and baking soda, the taste may become unpleasant. If baking soda is used instead of baking powder, the flavor may become bitter. Incorrect substitution of one for the other in recipes can also impact how quickly baked items rise.
Ques. Why it is essential to use both baking powder and baking soda? (2 marks)
Ans. In recipes where more leavening is required than acid present, both baking soda and baking powder may be used. For baking to work as best it must, the two leavening agents must be balanced. Their usage can also have an impact on baked items' flavor and browning.
Read More:








Comments