The CUET PG 2025 exam for Chemical, Thermal, and Polymer Engineering will be conducted from 13th May to 3rd June 2025. Post-exam, candidates will be able to access the question paper PDF, answer key, and solved solutions online. This exam tests your knowledge of chemical reaction engineering, heat and mass transfer, fluid mechanics, thermodynamics, polymer science, material science, and process control.
The test contains 75 objective questions, all to be answered in 60 minutes, for a total of 300 marks. Each correct response earns 4 marks, while 1 mark is deducted for every incorrect answer.
CUET PG Chemical, Thermal, and Polymer Engineering 2025 Question Paper with Answer Key PDF
| CUET PG Chemical, Thermal, and Polymer Engineering Question Paper with Solutions PDF | Download PDF | Check Solutions |

CUET PG Chemical, Thermal, and Polymer Engineering 2025 Question Paper with Solutions
The eigen value corresponding to the eigen vector ![]() for the matrix
for the matrix  is:
is:
For the function \(f(x) = 2x^3 - 15x^2 + 36x + 10\), the local maxima and local minima occur respectively at:
For an ordinary differential equation \( y'' + y' = x^2 + 2x + 4 \), the particular integral is given by:
Bag I contains 4 red and 3 green balls and Bag II contains 3 blue and 4 green balls. One ball is drawn at random from each bag, then the probability that one of the drawn balls is red and the other is blue, is:
Match the LIST-I with LIST-II:
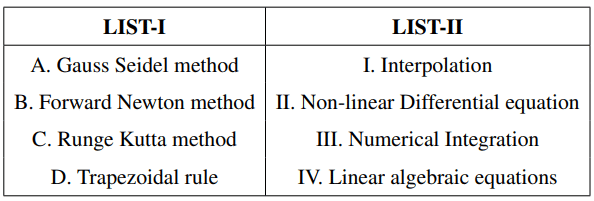
Choose the correct answer from the options given below:
In a constant pressure cake filtration with an incompressible cake layer, the volume of filtrate (V) is measured as a function of time, t. The plot of \( \frac{t}{V} \) versus \( V \) gives a straight line with slope \( 10^4 \, sm^{-3} \). Filter area is \( 0.005 \, m^2 \), viscosity of filtrate is \( 10^{-3} \, Pa.s \), and the overall pressure drop across filter is \( 200 \, kPa \). What is the value of filter medium resistance in \( m^{-1} \)?
A rigid spherical particle is undergoing free settling in a liquid of density 750 \( kg/m^3 \), viscosity \( 9.81 \times 10^{-3} \, Pa.s \). Particle density is four times the liquid density. Taking the acceleration due to gravity as \( 9.81 \, m/s^2 \) and assuming Stokes' law is valid, the terminal settling velocity of the particle in \( ms^{-1} \) is:
In the laminar boundary layer over a flat plate, the ratio of \( \delta/x \) varies as \( Re^k \) (Where, \( Re \) is Reynolds number). The value of \( k \) is:
The best purpose served by the baffles used in agitated vessels is to:
A pipe with diameter 25 cm at the entrance carries oil with specific gravity 0.9 at a velocity of 3 m/s. At the exit section the diameter of the pipe is 20 cm. The velocity at the exit section is:
Match LIST-I with LIST-II and select the correct answer using the codes given below the lists.
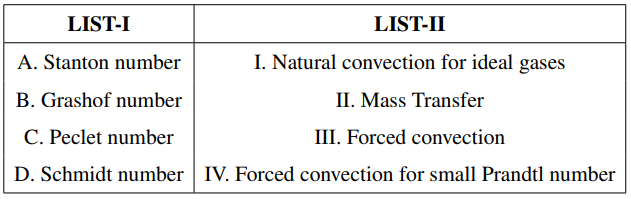
Choose the correct answer from the options given below:
Two rods, one of length L and the other of length 2L, are made of the same material and have the same diameter. The two ends of the longer rod are maintained at 100°C. One end of the shorter rod is maintained at 100°C while the other end is insulated. Both the rods are exposed to the same environment at 40°C. The temperature at the insulated end of the shorter rod is measured to be 55°C. The temperature at the mid point of the longer rod would be:
For steady flow and constant value of conductivity, the temperature distribution for a plane wall is:
Baffles are provided in heat exchangers to:
A furnace wall of thickness 1m and surface area 2m² is made of a material whose thermal conductivity is 1 kJ/hr.m°C. The temperature of the inner surface of the wall is 1000°C and of outer surface is 200°C. Heat flow through the wall in kJ/hr will be:
Match LIST-I with LIST-II and select the correct answer using the codes given below the lists.
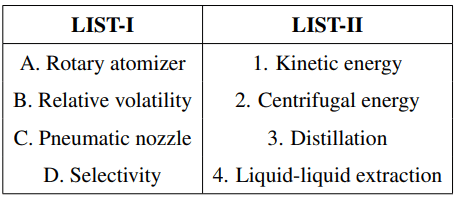
Choose the correct answer from the options given below:
The McCabe-Thiele method used to find the number of theoretical stages required in a distillation operation is based on the assumption(s) that:
Steady state temperature reached by a small amount of liquid evaporating into a large amount of unsaturated vapour-gas mixture is:
For economical operation in gas absorption, the operating line approximately:
Which of the following is used as an 'entrainer' in the separation of acetic acid-water mixture by distillation?
Compared to saturated oils, unsaturated oils have:
A production equipment costs ₹200000. Its salvage value is ₹25000. The expected return is ₹50000 per annum. The corporate taxes are taken as 30%. The payback period will be:
For a typical project, the cumulative cash flow is zero at the:
When the interest is compounded monthly, the effective interest rate will be:
For a shell and tube heat exchanger, the clean overall heat transfer coefficient is calculated as 250 Wm\(^2\)K\(^{-1}\) for a specific process condition. It is expected that the heat exchanger may be fouled during the operation and a fouling resistance of 0.001 m\(^2\)KW\(^{-1}\) is prescribed. The dirt overall heat transfer coefficient is ------------ Wm\(^2\)K\(^{-1}\).
In the industrial production of nitric acid, oxidation of ammonia with air in the presence of 90% platinum, 10% rhodium catalyst in the converter constitutes the first step, the feed gas to the converter contains around:
In forced convection, the Nusselt number (Nu) is a function of:
If a mass of solid particles is packed by tapping, the bulk density of the mass:
Consider separation of dust particles from gases in a cyclone separator. For a given particle size, the terminal velocity is maximum:
When a ball mill rotates at a speed higher than the critical speed, its efficiency is:
Which of the following is a coarse crusher?
Separation of particles of various sizes, shapes, and densities by allowing them to settle in a fluid is called:
Break-even point increases with:
Cost of instrumentation in a modern chemical plant as compared to the total plant cost, ranges from:
The rate of corrosion of mild steel by sulphuric acid is unacceptably high for:
Karbate is:
Bracket supports are most suitable for:
Consider a piston-cylinder arrangement containing a gas. This system is heated by placing it on the top of a burner. The system undergoes:
Consider the manufacturing of Linear Alkyl Benzene Sulphonates (LABS). The alkylbenzenes are sulphonated with:
In petrochemical industry, any petroleum fraction having an approximate boiling point range between 20°C and 200°C which is used as a feed stock is called:
As pressure approaches zero, the ratio of fugacity to pressure (f/P) for a gas approaches:
Secondary nutrients in fertilizers are:
The process involved in converting rubber into a thin sheet or coating it on fabric is called:
Mixed fertilizer having its grade designated by 5-25-10, means:
Sulfa drugs are derivatives of:
A multiple effect evaporator has a capacity to process 4000 kg of solid caustic soda per day when it is concentrating from 10% to 35% solids. The water evaporated in kg per day is:
Match LIST-I with LIST-II and select the correct answer using the codes given below the lists:

ABS is a:
The unit step response of the transfer function \( G(s) = \frac{1}{s^2 + 2s + 3} \)
has:
10 ml of 0.5 M HCl is mixed with 30 ml of 0.3 M HCl, the molarity of the solution is:
A first-order system with unity gain and time constant \( \tau \) is subjected to sinusoidal input having a frequency \( \omega \) of \( \frac{1}{\tau} \). The amplitude ratio for such system is:
Carothers equation may define gel formation for the:
A step change of magnitude is introduced to a system having the transfer function \[ G(s) = \frac{2}{s^2 + 2s + 4} \]
The percent overshoot is:
The Bode diagram stability criterion is applicable when:
In an internal combustion engine, during the compression strokes, the heat rejected to the cooling water is 75 J/g and the work input is 120 J/g. The change in internal energy of the working fluid is:
The functionality of the Phenol in the preparation of phenol-formaldehyde resin is :
The characteristic equation for the system is \[ s^3 + 9s^2 + 26s + 12(2 + K_c) = 0 \]
Using Routh test, the value of Kc that will keep the system stable is:
Which one is an example of branched polymer?
The transfer function of a PID controller is:
The liquid used in hydraulic transmission is:
Bode diagram is a plot of (AR is amplitude ratio; \(\omega\) is frequency factor and \(\phi\) is phase angle):
Which one is appropriate for the poly-ethyleneterephthalate?
Match the LIST-I with LIST-II
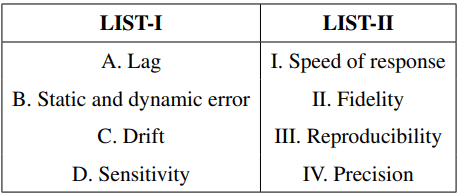
Choose the correct answer from the options given below:
Unit of rate constant for a zero order reaction is:
If the temperature of a saturated water is increased infinitesimally at constant entropy, the resulting state of water is:
Buna-S is a polymer of:
Accuracy is specified as 0.5% of the true value. At 5% of full scale, error of the instrument will be:
Water enters a storage tank at a temperature, \( T_0 \) and flow rate, \( Q_0 \) and leaving at a flow rate, \( Q \) and temperature, \( T \). There are negligible heat losses in the tank. The area of cross-section of the tank is \( A_c \). The model equation that describes the water temperature variation in the tank is given by:
HDPE water storage tank is an example of:
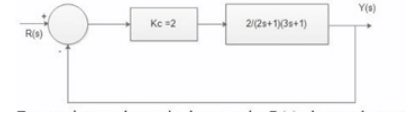
The Block diagram for a control system is shown below:
A gaseous system contains \( H_1 \), \( H_2 \), and \( I_2 \) which participate in the gas phase reaction: \[ 2H_1 \rightleftharpoons H_2 + I_2 \]
At a state of reaction equilibrium, the number of thermodynamic degrees of freedom is:
From the following list, identify the properties which are equal in both vapor and liquid phases at equilibrium:
(A) Temperature
(B) Density
(C) Enthalpy
(D) Chemical potential
Choose the correct answer from the options given below:
For a pure substance, the Maxwell's relation obtained from the fundamental property relation \( dU = T dS - P dV \) is:
For a pure substance, which of the following does not change during condensation?
Which of the following conditions is not satisfied by an ideal solution?



Comments