
Content Curator
Oxalate is an ionic form of a Oxalic acid (a toxic acid) that is formed in our body from a combination of various food sources and their absorption in the gastrointestinal tract. These Oxalate crystals block the absorption and utilization of Calcium in the body, causing diseases such as Rickets and Osteomalacia, which are diseases related to weakening of bones.
| Table of Content |
Keyterms: Oxalate, Oxalic Acid, Toxic acid, Calcium, Rickets, Osteomalacia, Bones, Absorption, Food, Spinach, Beets, Peanuts, Rhubarb, Chocolate, Sweet potatoes
Sources of Oxalate
Oxalate is naturally found in many foods, including fruits and vegetables, seeds and nuts, grains, legumes, and even tea and chocolate. Some examples of foods that have more levels of oxalate include: spinach, beets, peanuts, rhubarb, chocolate and sweet potatoes.
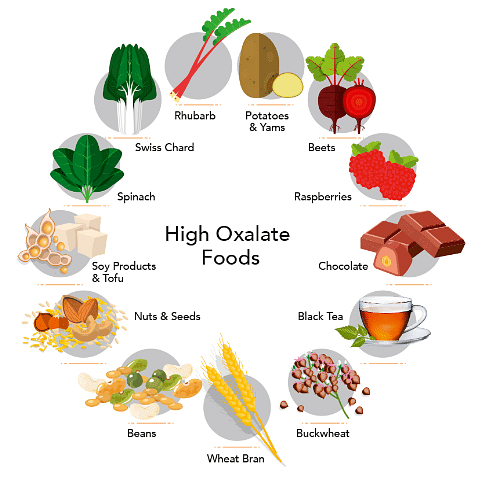
Fig. High Oxalate Foods
Removal of Oxalate
- Drinking plenty of water to helps flush oxalates out of the body.
- Consuming enough calcium can bind oxalates during digestion
- Limit the consumption of sodium and sugar in food.
- Consume the recommended amounts of vitamin C, not in excess.
- Cooking certain vegetables can lessen their oxalate content.
How is Oxalate Formed?
Oxalates are formed by Deprotonation. It is widely used for derivatives, such as salts of oxalic acid, for example, dimethyl Oxalate or sodium Oxalate. Oxalate also forms coordination compounds and is at times abbreviated as “ox”.
De-protonation is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction] of both the carboxy groups of C2H2O4 (oxalic acid).
Explore more on Acids: Acids, Bases, and Salts
Chemical Formula and Structure of Oxalate
Chemical Formula:
Chemical formula of Oxalate is C2O4-2
Structure of Oxalate Molecule:
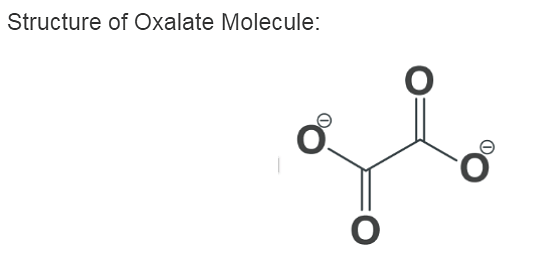
In X-ray Crystallography, simple Oxalate salts appear as a planar conformation with a D2h molecular symmetry, and with a conformation where the O–C–C–O, dihedrals approach at a 90° angle along with an approximate D2d symmetry.
Read more: Atoms and Molecules.
Uses of Oxalate
Although natural Oxalates are harmful to the human body, artificially made oxalates are useful to mankind in many ways.
- Calcium Oxalate is used in the production of ceramic materials,
- Barium Oxalate and Strontium Oxalate are used as reducing agents in laboratory chemistry and pyrotechnics.
- Potassium hydrogen-Oxalate, also known as salt of sorrel, is used as chemical reagents due to its occurrence in the sorrel plant. It is also used in photography and for the removal of ink stains.
- Ferric Oxalate is a chemical compound used in platinum printmaking,
- Cobalt Oxalate is used in the production of cobalt catalysts.
- Oxalates may also be used in some instances as sources of the metals they contain.
Profile of Oxalate
The profile of Oxalate has been tabulated below:
| Molecular weight | 88.019 gm/mol |
| Number of Hydrogen bond acceptors | 4 |
| Mono isotopic mass | 87.98 g/mol |
| Number of Hydrogen bond donors | 0 |
Effects on Health
Excess intake of foods containing high amounts of Oxalic acid can show adverse effects on Health. Oxalic acid present in the food combines with divalent metallic cations such as iron (II) and calcium and form crystals of the corresponding Oxalates. These crystals are then excreted from urine as minute crystals.
These Oxalates may result in the formation of larger kidney stones which can obstruct the kidney tubules. Approximately 80% of kidney stones are formed due to calcium Oxalate. Foods that contain high amounts of Oxalates should not be consumed raw. They should be boiled or soaked in water before consumption. Mostly sufficient intake of water everyday can drive out Oxalates out of the body and reduce the risk of kidney stones.
Things to Remember
- Oxalate is an ionic form of a Oxalic acid, that is formed in our body from a combination of various food sources and their absorption in the gastrointestinal tract.
- Excess of oxalate content in the body can cause few serious health problems like formation of kidney stones
- Chemical formula of Oxalate is C2O4-2
- Drinking plenty of water, consuming enough calcium, limiting sodium and sugar intake, consuming recommended amount vitamin c, and cooking certain vegetables can prevent the formation oxalates in our body.
- Various forms of Oxalates are used in manufacture of ceramic materials, reagents in laboratories, removal of ink stains and photography, production of cobalt catalysts, etc.
Sample Questions
Ques. What electrical charge does the Oxalate ion possess? (2 Marks)
Ans. C2O4 is a polyatomic ion with a -2 electrical charge. In this molecule, the oxygen atom has an oxidation state of -2 as oxygen also has a -2 charge on it.
Ques. Is Oxalate a required nutrient for people? (2 Marks)
Ans. Although Oxalate is not a required nutrient for the human body, it is consumed unknowingly through foods, but excess consumption of oxalate rich foods can result in the formation of kidney stones.
Ques. What foods have no Oxalates? (2 Marks)
Ans. Foods such as Artichokes, asparagus, bamboo shoots, cauliflower, chayote squash, chicory, corn, cucumbers, endive, lettuce, lima beans, broccoli, brussels sprouts, cabbage, mushrooms, onions, peas, peppers, potatoes, radishes, rutabagas, zucchini have no Oxalates.
Ques. What is the pH of Oxalate? (2 Marks)
Ans. The risk of calcium Oxalate (CaOx) crystallization at different pH levels was determined in urine from recurrent CaOx-stone formers and normal subjects. The highest risk of crystallization was observed between pH 4.5 and 5.5.

Fig. Oxalate Ion
Ques. Is rice rich in Oxalates? (2 Marks)
Ans. Some of the basic ingredients to make certain foods are very high in oxalates. White flour and brown rice flour are high in Oxalate, and so any product made from them will be high in oxalates. But on cooking, oxalates in the rice are diluted. Oxalates can be reduced in our body by taking calcium rich foods, drinking plenty of water every day, and avoiding excess intake of table salt and sugar in the food.
Ques. What is an Oxalate ion? (3 Marks)
Ans. Oxalate is an organic acid found in plants, but can also be synthesized by the body. It binds minerals, and has been linked to kidney stones and other health problems. Oxalate ion is also called Ethanedioate or Dianion of Oxalic Acid.
This is obtained in our body by de-protonation of both C2H2O4 (oxalic acid) carboxy groups. It is commonly used, for instance, for compounds such as oxalic acid salts, di-methyl Oxalate, or sodium Oxalate.
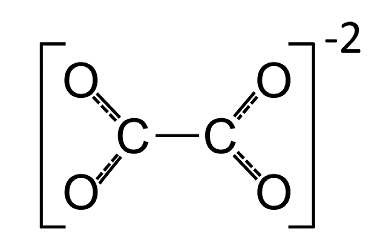
Fig. Molecular Structure of Oxalate Ion
Ques. Why does the body produce Oxalate? (3 Marks)
Ans. Human body can produce Oxalate on its own or obtain it from food. Vitamin C is converted into Oxalate when it's metabolized. Once consumed, Oxalate can bind to minerals to form compounds, including calcium Oxalate and iron Oxalate
Oxalate may be absorbed in our body passively at any site along the gastrointestinal tract even though the main sites of absorption appear to be in the stomach and in the colon. Absorption of Oxalate is influenced by the concentration of other dietary constituents such as calcium, magnesium and fiber.
Ques. How long does it take to flush Oxalates? (3 Marks)
Ans. One way to slow down deposit of waste is to limit intake of Oxalate-rich foods. Driving out of excess Oxalates can take up to a year in some cases.
Dietary and lifestyle choices can also reduce the impact of oxalates:
- Drinking plenty of water to help your body flush oxalates out.
- Limiting sodium and sugar intake, which may contribute to kidney stones at high levels.
- Getting the recommended amounts of vitamin C — too much can increase oxalic acid production in your body.
- Cooking some vegetables can lower their oxalate content.





Comments