UP Board Class 12 Chemistry Question Paper 2025 PDF (Code 347 KF) is available for download here. The Mathematics exam was conducted on March 8, 2025 in the Morning Shift from 2:00 PM to 5:15 PM. The total marks for the theory paper are 100. Students reported the paper to be easy to moderate.
UP Board Class 12 Chemistry Question Paper 2025 (Code 347 KF) with Solutions
| UP Board Class Physics Question Paper with Answer Key | Check Solutions |

UP Board Class 12 Chemistry Questions with Solutions
The aqueous solution having maximum boiling point is
A colourless ion in the following is
The oxidation number of Cu in the ion [Cu(CN)\(_4\)]\(^{3-}\) is
Rosenmund reduction gives
How many primary amines are possible for the formula C\(_4\)H\(_{11}\)N?
How many primary alcoholic groups are there in glucose?
((a) 5.85 g of NaCl is dissolved in 200 ml water. Calculate the molarity of the solution. [Na = 23, Cl = 35.5]
(b) In comparison to Mn\(^ {3+}\) ions Mn\(^ {2+}\) ions are more stable. Why?
(c) Differentiate between double salt and complex salt by giving two examples of each.
(d) Write the equation of any one of nucleophilic substitution reactions of ethyl bromide.
(a) At what temperature does a 5% (w/v) solution of glucose produce 7 atmospheric osmotic pressure? \([ R = 0.0821 \, L \cdot atm / K mol ]\)
(b) Write the chemical equation for the preparation of phenol.
(c) \(pK_b\) value of aniline is more in comparison to that of methyl amine. State the reason.
(d) Write a note on Tollen’s test.
(a) Which one between 0.1 M Urea and 0.1 M NaCl solution will have greater osmotic pressure? Explain the reason.
Explain the redox potential.
Explain the zero order of reaction by giving examples.
Write the color of following metal ions in aqueous solution:
(a) Calculate the electromotive force of the following cell:
\[ Cu | Cu^{2+} (1M) || Ag^{+} (1M) | Ag \]
Given \( E^\circ_{Cu^{2+}/Cu} = +0.34V \), \( E^\circ_{Ag^{+}/Ag} = +0.80V \)
Explain the molecularity of reaction with example.
Explain that \([Co(NH_3)_5Cl]SO_4\) and \([Co(NH_3)_5SO_4]Cl\) are of which type of isomers.
Fehling’s solution is used to identify which type of compounds? Write chemical equations.
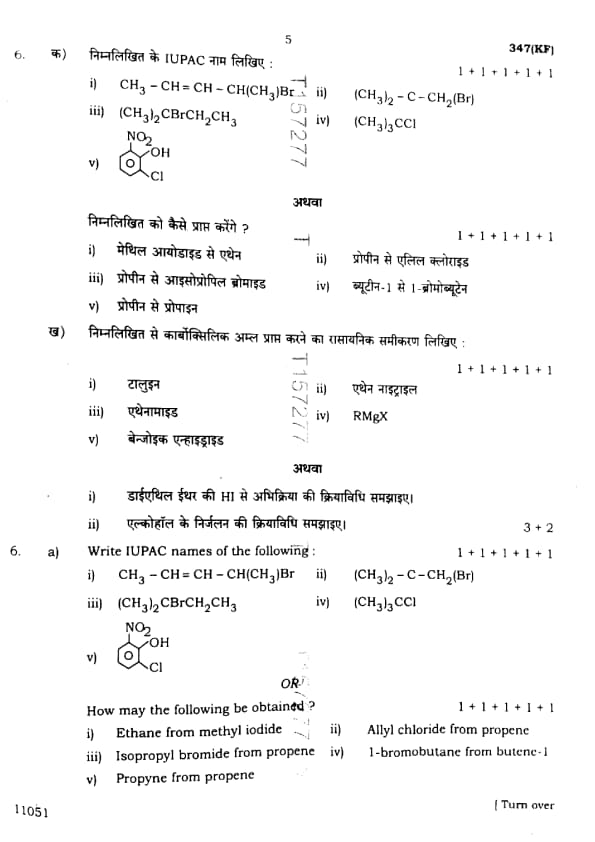
(a) Write IUPAC names of the following:
How may the following be obtained?
Write chemical equations for obtaining carboxylic acid from the following:
Explain the mechanism of the reaction of diethyl ether with HI.
Explain the mechanism of dehydration of alcohol.
(a) Write the chemical equation for the preparation of acetaldehyde and write a note on Aldol condensation and cross-Aldol condensation.
Write notes on the following:
(a) Mechanism of esterification of carboxylic acid:
Write the chemical equation for the preparation of Aniline and write the chemical equation of its reaction with the following:
Write short notes on the following:
(a) Coupling reaction:









Comments