Muskan Shafi Education Content Expert
Education Content Expert
Mendeleev’s Periodic Table is an arrangement of 63 known elements discovered till 1869 in a tabular format according to their properties. The elements are arranged according to the increasing atomic mass and chemical characteristics in Mendeleev’s Periodic Table. In Mendeleev's periodic table, the elements were arranged in a horizontal manner in 10 series. These series were further divided into 7 horizontal columns known as periods and 8 vertical columns known as groups.
Dmitri Ivanovich Mendeléev, a Russian chemist, discovered Mendeleev’s Periodic table after Newlands Octave Law was rejected in 1869. He arranged the elements so that the ones with similar properties come under the same vertical columns of his periodic table.
Read More: Periodic Properties of Elements
Key Terms: Mendeleev’s Periodic Table, Atomic Mass, Elements, Newlands Octave Law, Periodic Table, Elements
Mendeleev’s Periodic Table
[Click Here for Sample Questions]
Mendeleev’s Periodic Table is based on the law that the properties of elements are the periodic function of their atomic masses.
- Mendeleev's Periodic Table was introduced in 1869. Elements were arranged in this table according to their atomic mass.
- Only 63 elements were known at Mendeleev's time.
- After analyzing their qualities, Mendeleev discovered that the properties of elements were related to atomic mass in a periodic pattern.
| Mendeleev’s Periodic Law: “Properties of elements are the periodic function of their atomic masses.” |
- Mendeleev considered formulae of hydrides and oxides to be one of the most basic criteria for categorization among chemical qualities.
- He took 63 cards and wrote the properties of one element on each one. He pinned the elements that have similar qualities together on the wall.
- He saw that elements were ordered in increasing order of atomic mass and that elements with comparable properties appeared on a regular basis.
- The elements were placed horizontally in 10 series in Mendeleev's periodic table in order of increasing atomic weights.
- Seven horizontal columns (period) and eight vertical columns were used to split these series (groups).
Mendeleev's periodic table was changed as the new elements were discovered, and the new elements were then added to the appropriate positions to render the modern periodic table.
Read More:
Mendeleev’s Periodic Table Elements
[Click Here for Previous Years' Questions]
The elements in Mendeleev’s Periodic Table are as given below:
| Element | Symbol | Atomic Mass |
|---|---|---|
| Hydrogen | H | 1.01 |
| Lithium | Li | 7 |
| Beryllium | Be | 9.4 |
| Boron | B | 11 |
| Carbon | C | 12 |
| Nitrogen | N | 14 |
| Oxygen | O | 16 |
| Fluorine | F | 19 |
| Sodium | Na | 23 |
| Magnesium | Mg | 24 |
| Aluminum | Al | 27 |
| Silicon | Si | 28 |
| Phosphorus | P | 31 |
| Sulphur | S | 32 |
| Chlorine | Cl | 35.5 |
| Potassium | K | 39 |
| Calcium | Ca | 40 |
| Vanadium | V | 51 |
| Chromium | Cr | 52 |
| Manganese | Mn | 55 |
| Iron | Fe | 56 |
| Cobalt | Co | 59 |
| Nickel | Ni | 59 |
| Copper | Cu | 63.4 |
| Zinc | Zn | 65.2 |
| Titanium | As | 50 |
| Selenium | Se | 78.96 |
| Bromine | Br | 79.904 |
| Rubidium | Rb | 85.4678 |
| Strontium | Sr | 87.6 |
| Yttrium | Y | 88.90585 |
| Zirconium | Zr | 91.224 |
| Niobium | Nb | 92.90 |
| Molybdenum | Mo | 95.94 |
| Ruthenium | Ru | 101.07 |
| Rhodium | Rh | 102.9 |
| Palladium | Pd | 106.42 |
| Silver | Ag | 107.86 |
| Cadmium | Cd | 112.41 |
| Indium | In | 114.81 |
| Tin | Sn | 118.71 |
| Seaborgium | Sb | 121.76 |
| Tellurium | Te | 127.6 |
| Iodine | I | 126.90 |
| Cerium | Ce | 140.11 |
| Barium | Ba | 137.32 |
| Lanthanum | La | 138.90 |
| Tantalum | Ta | 180.94 |
| Tungsten | W | 183.84 |
| Osmium | Os | 190.23 |
| Iridium | Ir | 192.21 |
| Platinum | Pt | 195.07 |
| Gold | Au | 196.96 |
| Mercury | Hg | 200.59 |
| Lead | Pb | 207.2 |
| Bismuth | Bi | 208.98 |
| Thallium | Th | 204.38 |
| Uranium | U | 238.02 |
Characteristics of Mendeleev's Periodic Table
The properties of Mendeleev’s Periodic Table are:
- The items in this table are organized into vertical columns named groups and horizontal rows called periods.
- I, II, III, IV, V, VI, VII, VIII, and 0 are the nine groupings identified by Roman numbers.
- The elements in the first seven groups have been separated into two subgroups, A and B, based on their similar qualities.
- Subgroup A is made up of items on the left side of each group, while subgroup B is made up of elements on the right side.
- Group VIII is made up of nine elements organized in triads. The zero group is made up of elements that belong to inert (noble) gases and have zero valencies.
- There are seven distinct periods (numbered from 1 to 7) in Mendeleev Periodic Table.
- Periods 4,5,6, and 7 are divided into two halves to allow more elements.
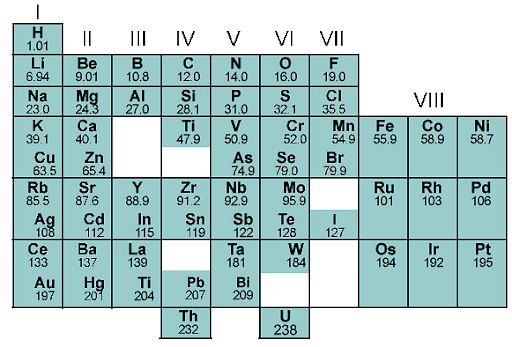
Mendeleev Periodic Table of Elements 1869
- In each box, the first half of the elements are placed in the upper left corner, while the second half is placed in the lower right corner.
- The shortest period is the first one with only two elements. Short periods are the second and third periods, each of which has eight elements.
- Long periods are those with eight elements and are classified as such by the 4th and 5th categories.
- The 6th period of the periodic table, which has 32 elements, is considered the longest.
Read More:
Merits of Mendeleev's Periodic Table
[Click Here for Previous Years' Questions]
The advantages of Mendeleev’s periodic table are:
Systematic Study of Chemistry
- For the first time, Mendeleev's periodic table categorized the elements in a symmetrical manner.
- The properties of other elements and their compounds in the group can be inferred by knowing the properties of one element.
- This simplified the study of elements.
Prediction of New Elements
- Only 63 elements were known when Mendeleev's periodic table was created.
- As a result, Mendeleev omitted specific vacant spaces while ordering the components according to their properties.
- Unknown elements were represented by these gaps.
- Furthermore, based on their positions, Mendeleev anticipated the properties of these undiscovered elements.
- Gallium and germanium, for example, were unknown at the time of Mendeleev’s periodic table.
- These elements were given the names Eka-aluminium and Eka-silicon by Mendeleev because he thought their properties would be similar to those of aluminum and silicon, respectively.
- The properties of Eka-aluminium discovered and named gallium by De Baisbaudron, and Eka-silicon, discovered and named germanium by Winkler, are the same as those predicted by Mendeleev.
Correction of Atomic Masses
- Mendeleev's periodic chart was used to calculate the correct atomic masses of numerous elements.
Demerits of Mendeleev's Periodic Table
[Click Here for Sample Questions]
Mendeleev’s periodic table also has some disadvantages which are:
Position of Hydrogen
- Since hydrogen possesses both alkali metal (IA group) and halogen characteristics (VIIA group), it should have been assigned to both groups IA and group VIIA based on its qualities.
- However, in this periodic table, hydrogen is assigned to the IA group.
Position of Isotopes in Mendeleev's Periodic Table
- Elements are organized in increasing atomic masses in Mendeleev's periodic table.
- As a result, various isotopes of an element should be placed in distinct locations.
- However, because isotopes have comparable chemical properties, this will be inconsistent.
- Protium, deuterium, and tritium, the isotopes of hydrogen, with atomic weights of 1, 2, and 3, should be stored in separate locations.
- These, however, are not listed in the periodic table at separate times.
Lanthanides and Actinides
- Lanthanides are the 14 elements with atomic numbers 58 to 71 that follow lanthanum.
- Actinides are the 14 elements with atomic numbers 90 to 103 that follow actinium.
- As they do not follow Mendeleev's periodic law, these arrangements are anomalous in Mendeleev's periodic table.
Variable Valency Elements
- A considerable number of elements have variable valencies.
- However, Mendeleev's periodic table made no mention of this.
Anomalous Pair of Elements
- Several elements are not arranged according to Mendeleev's periodic law, i.e., they are not arranged in the order of increasing atomic mass.
- Argon (atomic number 39.9), for example, is placed before potassium (atomic number 39.1).
- Tellurium (atomic mass =127.61) is also placed ahead of Iodine (atomic mass =126.91).
- The potassium should be placed before the argon if the atomic weight rule is carefully observed.
- Potassium belongs in the zero group, whereas argon belongs in the IA group.
Similar Elements not Placed in the Same Groups
- Elements with similar qualities, such as silver and thallium, barium and lead, copper and mercury, are placed in separate groups in Mendeleev's periodic table.
Dissimilar Elements are Grouped Together
- The elements in a group's subgroups A and B have different physical and chemical properties, yet they are nevertheless been classified as the same group in Mendeleev's periodic table.
- Except for osmium, no other element in this group has group valency, which means it is octavalent. Anomalies exist in atomic weight and a number of other attributes.
Reason for Periodicity
- No satisfactory explanation has been presented for why the attributes of components placed in a group are similar.
- The Modern Periodic Table corrected the flaws in Mendeleev's Periodic Table.
Things to Remember
- Dmitri Ivanovich Mendeléev, a Russian chemist, is credited with the development of Mendeleev’s periodic table.
- Only 63 elements were known when Mendeleev’s periodic table was created.
- Mendeleev arranged elements according to their fundamental property, atomic mass, and chemical properties.
- According to Mendeleev’s periodic law, the properties of elements are the periodic function of their atomic masses.
- Mendeleev left some gaps in his periodic table for the elements which were yet to be discovered.
- However, he was not able to locate the position of hydrogen in the periodic table.
- Isotopes of elements were also discovered later that violated Mendeleev’s periodic law.
Sample Questions
Ques: What is the Mendeleev periodic table law? (2 Marks)
Ans: Mendeleev declared in his famous periodic law that “Element characteristics are a periodic function of their atomic masses". The Periodic Table of Mendeleev was created by Mendeleev, who arranged elements in order of their atomic weights.
Ques: List the demerits of Mendeleev’s periodic table. (3 Marks)
Ans: The three major demerits of Mendeleev’s periodic table are:
- Mendeleev was unable to locate the position of hydrogen in the periodic table.
- The increase in atomic mass was not periodic while moving from one element to another. Therefore, it was not predictable to know the number of elements yet to be discovered.
- Isotopes of elements were found later on which violated Mendeleev’s periodic law.
Ques: How did Mendeleev arrange the elements in his periodic table? (5 Marks)
Ans: Mendeleev, on studying the properties of every element, found that the properties of elements were a periodic function of their atomic mass. He then arranged the elements in such a manner that the elements with similar properties fell into the same vertical columns of the periodic table.
For the chemical properties, he considered hydrides and oxides as one of the basic criteria for classifying the elements. He took 63 cards and wrote the properties of each element on one card and then grouped the elements with similar properties and pinned it on the wall. In this manner, he made two observations. First, the elements were arranged in the increasing order of atomic mass and second, there was the periodic occurrence of elements with similar properties.
Ques: What's the difference between Mendeleev's periodic table and the modern one? (3 Marks)
Ans: The primary distinctions are that Mendeleev's periodic chart is based on atomic mass. The current periodic table is organized according to the number of atoms in each element. Noble gases were not included in Mendeleev's periodic table because they were not discovered at the time. Noble gases are classified as group-18 in the modern periodic table.
Ques: Mention the advantages of Mendeleev’s periodic table. (3 Marks)
Ans: The advantages of Mendeleev’s periodic table are as follows:
- In case any inert or noble gas would be discovered later then it could be placed in a separate group. The noble gases were placed in different groups because these gases are chemically not reactive.
- Mendeleev made sure that enough spaces were left vacant to place the future elements that will be discovered.
Ques: How did Mendeleev discover the mass of an atom? (2 Marks)
Ans: Mendeleev was not the one who calculated the atomic mass. He had the data he needed to look for repeating qualities and a pattern that turned out to be a chart. On the basis of the pattern he noticed for the atoms that were not detected, he projected their atomic mass.
Ques: Explain the arrangement of elements in Mendeleev’s periodic table. (5 Marks)
Ans: In Mendeleev’s periodic table, the elements were arranged in the periodic table in the increasing order of their relative atomic masses.
- Mendeleev divided the periodic table into 8 groups and 7 periods.
- Groups I to VII were for normal elements and group VIII was for transition elements.
- Groups I to VII was divided into two subgroups, while group VIII was meant for three elements.
- Periods from 4th to 7th were divided in two series namely the 1st series and the 2nd series.
Ques: What is Mendeleev's claim to fame? (2 Marks)
Ans: Mendeleyev is most known for developing the periodic law and the periodic chart of elements, which he adopted in 1869. He died in St. Petersburg, Russia, on February 2, 1907.
Ques: In the periodic table, where do you look for electrons? (2 Marks)
Ans: The number of protons, neutrons, and electrons in an atom is determined by a set of basic laws. The number of protons in the hydrogen nucleus equals the atomic number (Z). The number of electrons in a neutral atom equals the number of protons.
Ques: What was Mendeleev's reasoning for leaving gaps in the Periodic Table? (2 Marks)
Ans: Mendeleev placed holes in the Periodic table for elements that had yet to be discovered, such as scandium, germanium, and gallium.
Study Guide Links:



Comments