
Collegedunia Team Content Curator
Content Curator
Basic law that governs the modern periodic table states that the properties of the elements are periodic functions of their atomic number. Periodic Properties of Elements reappear at regular intervals or follow particular or follow the particular trend at regular intervals.
Read More: Rutherfordium
| Table of Content |
Keyterms: modern periodic table, elements, Periodic Properties of Elements, atom, shell, electrons, velocity
Read More: Ruthenium
Introduction to Periodic Properties
[Click Here for Sample Questions]
Periodic properties of elements are determined by the valency and the number of shells in an atom. As we move down a group the number of shells increases successively such that the number of the shell of an element is equal to the number of periods to which it belongs.
As we move across a period the number of shells remains the same. For instance elements of the second period have two shells.

Periodic Properties
Valency is the combining capacity of an atom. It is equal to the number of electrons that an atom can accept or donate in order to complete its octet. As one moves down a group the number of electrons in the valence shell remains the same, this means that the valency of a group is constant.
Valency is highly dependent on the number of electrons in the outermost shell of an atom. Because the combining capacity of an atom is called valency it will always have a positive value and impact the periodic properties.

Periodic Properties
Atomic Radius
Half the distance between the centers of two atoms of an element that are just touching each other is called the atomic radius of an element. It is known to decrease across a period from left to right and increase down a given group. The atoms that have the largest atomic radii are located in group 1 and at the bottom of the groups.

Atomic Radius
Ionization Energy
The ionization potential is the energy required to remove an electron from a gaseous atom or iron completely. The closer and tighter the bond of an electron is to the nucleus the more difficult it is to remove and the higher its ionization energy will be.
The first ionization energy is energy that is required to remove an electron from the parent atom. The second ionization energy is energy which is required to remove the S valence electron from the univariate ion to form the divalent ion and this goes on.
Successive ionization energies tend to increase. This increases moving from left to right across a period and decreases as one moves down during a group. Group 1 elements have low ionization energy because the loss of an electron forms a stable octet.

Ionization Energy
Electron Affinity
The ability of an atom to accept an electron is called Electron Affinity. Atoms that have stronger effective nuclear charge tend to have greater electron affinity. Some generalizations can be made out about the electron affinities of certain groups in the periodic table.
The elements that are relatively stable such as the alkaline earth have low electron affinity values as they are filled with subshells. Elements of other groups have low electron affinities. Electron affinity decreases as one moves down a group because of a new electron to be further from the nucleus of a large atom.
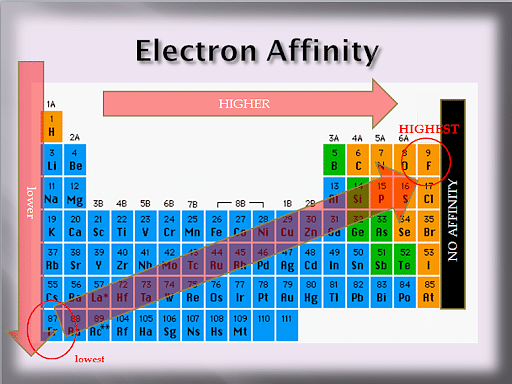
Electron Affinity
Electronegativity
Electronegativity is a measure of the attraction of an atom for electrons in a chemical bond. As the electronegativity of an atom increases the attraction for bonding electrons becomes greater.
It is related to ionization energy as they have low electronegativities because their nuclei do not exert a strong attractive force on electrons. High ionization energy has high electronegativity. The electronegativity decreases as the atomic number increases in a group. This results in the increased distance between valence electrons and the nucleus.

Electronegativity
Importance of Periodic Table
[Click Here for Previous Year Questions]
All the periodic table is described as an arrangement of elements in increasing mass numbers order, it provides the number of neutrons, protons, and average molar mass, the relative mass of an element which is used in chemistry calculations.
It acts as a list of all the elements that have been discovered to exist to date. Other than this it gives us an idea of whether an element is a metal or nonmetal. The trends in the periodic table and their arrangement into groups and periods help anyone to identify various trends across those elements.
Read More: Rubidium
Uses and Applications of Periodic Table
[Click Here for Sample Questions]
- Periodic table informs us about what atoms are more reactive to that of others as per their relative positions and compounds that hold the strongest bonds.
- The table provides an explanation for ionic and covalent bonding.
- It tells of the symbol, name, nucleon number, and atomic number that gives us an idea about the number of protons, electrons, neutrons, and several phenomena that can explain the use of three values.
Also Read:
Things to Remember
- Periodic table offers ways of understanding how to balance equations and their valency easily by looking at the groups where the atoms fall.
- The periodic table is of high value in the history of chemistry; it is based on Mendeleev Mosley’s periodic law. It gives a clear description of the properties of all chemical elements like the function of the atomic numbers or the proton numbers.
- It is composed of periods and groups. Setting about the periodic table is a good basis for understanding the whole inorganic chemistry and several properties as well as organic chemistry.
Important PYQs Related To This Chapter
- KF combines with HF to form… (BITSAT 2005)
- Which of the following has zero net dipole moment… (BITSAT 2019)
- In the clathrates of xenon with water…(BITSAT 2019)
- Which one of the following statements is not correct...M(AP EAPCET 2009)
- The chief component of cement is… (JIPMER 2000)
- The molecular electronic configuration of… (BITSAT 2005)
- Although the details of the structure of monoclinic sulphur… (CBSE CLASS XII)
- The element with the atomic number 118, will be… (NEET 1996)
- Which of the following eletronic configuration is not possible... (WB JEE 2018)
- The state of hybridization of B in… (AP EAPCET 1998)
- Which of the following element has the highest ionisation enthalpy… (BITSAT 2019)
- Although CO is neutral gas, yet it shows acidic nature … (JIPMER 2000)
- The cyanide ion CN− and N2 are isoelectronic...(JEE ADVANCED 1997)
- A certain compound X when treated with copper… (NEET 1994)
Sample Questions
Ques. Explain the cause of periodicity. (1 Mark)
Ans. The cause of periodicity is the reactions of similar electronic configuration i.e., the same number of electrons in the outermost orbit. In a particular group electrons in the outermost orbit remain the same which means electronic configuration is similar.
Ques. How can the valency of an element be determined if its electronic configuration is known? (2 Marks)
Ans. If the element has 1, 2, 3, 4 valence electrons, its valency will be 1, 2, 3, 4 respectively. If the element has 5, 6, 7, 8 valence electrons, its valency will be 3, 2, 1, 0. Element with atomic number 9 has electronic configuration 2, 7. So, its valency will be 1.
Ques. State the characteristics of the Moseley periodic table? (2 Marks)
Ans. The periodic table is a tabular array of the chemical elements organized by the atomic number from the element with the lowest atomic number hydrogen to the highest atomic number oganesson. The atomic number of elements is the number of protons in the nucleus of an atom of that element.
Ques. What were the two criteria used by Mendeleev in creating his Periodic Table? (2 Marks)
Ans. The two criteria that Mendeleev used to classify the element in the periodic table are:
- Increasing order of atomic mass is a physical property and similarities in chemical properties of elements.
- The formula and nature of hydride and oxide formed by the element were treated as basic chemical properties for its classification.
Ques. List two observations that post the challenge to Mendeleev’s periodic law. (3 Marks)
Ans. Following are the two observations that poster challenge to Mendeleev periodic law:
- Increasing order of atomic weights could not be maintained by matching chemical properties that do not depend upon atomic mass.
- Isotopes have different atomic masses but the same chemical properties.
Ques. What is Electron Affinity? (3 Marks)
Ans. Electron affinity is defined as the amount of energy released when an electron is added to a neutral atom to form an anion. In other words, it is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion.
So the more negative the electron affinity the more favorable the electron addition process is. Not all elements form stable negative ions in which case the electron affinity is zero or even positive. It is measured by dividing the Atomic size by 1.
Ques. How is the periodic table affected by moving from left to right and top to bottom? (3 Marks)
Ans. Moving Left→Right
- Atomic radius decreases
- Ionization energy increases
- Electron affinity generally increases
- Electronegativity increases
Moving Top→Bottom
- Atomic radius increases
- Ionization energy decreases
- Electron affinity generally decreases moving down the group
- Electronegativity decreases
Ques. Explain the periodic table properties of elements. (4 Marks)
Ans. On the periodic table elements with similar properties are in the same groups vertically. From left to right the atomic numbers of the elements increase from one period to the other horizontally. The group 7 number at the top of each column and periods on the left next to each row. Some of the important criteria as of the periodic properties of elements include:
- Atomic radius
- Ionization energy or ionization potential
- Electron affinity
- Electronegativity
- Metallic character
- Redox potentials
- Oxidation potential
- Reduction potential
Read more:





Comments