Muskan Shafi Education Content Expert
Education Content Expert
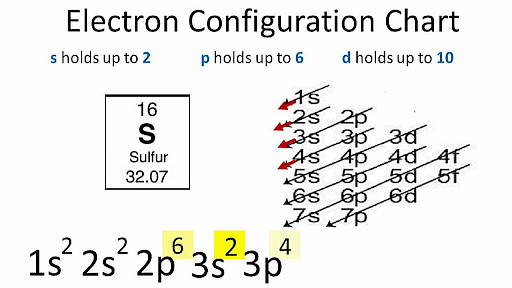
Electron Configuration describes how the electrons are distributed in an atom's orbitals. The electrons are arranged in four subshells namely s,p, d and f. The electron configuration of each atom is represented by following a standard notation. For example, the electron configuration of Sodium is 1s22s22p63s1. Here, all the electron-containing subshells with their number of electrons in superscript are written in a sequence.
Read More: Hybridisation
Key Terms: Electron Configuration, Electrons, Subshell, Atomic Orbitals, Quantum Number, Aufbau Principle, Hund’s Rule, Electronic Configuration
Electron Configuration
[Click Here for Sample Questions]
- Electron configuration describes how electrons are distributed in its atomic subshell.
- Atomic electron configurations follow a standard notation in which all electrons containing atomic subshells are placed in a sequence (with the number of electrons they hold written in superscript).
- For example, the electron configuration of sodium is 1s2 2s2 2p6 3s1.
- An electron in an atom is defined by a set of four quantum numbers (n), the most important of which defines the main energy level known as a shell.
- The position of an element in the periodic table is determined by the quantum numbers of the last orbital filled.
Electron Configuration
- For large atomic numbers, the standard notation of electron configuration may be very long. In such cases, instead of the standard notation, an abbreviated or condensed notation may be used.
- The sequence of completely filled subshells that correspond to the electronic configuration of a noble gas is replaced with the symbol of that noble gas in square brackets in the abbreviated notation.
- As a result, sodium's abbreviated electron configuration is [Ne]3s1 (the electron configuration of neon is 1s2 2s2 2p6, which can be abbreviated to [He]2s2 2p6).

Filling up of orbitals
Read More:
Writing Electron Configurations
[Click Here for Previous Year Questions]
The important terms that are related to electron configuration writing are as follows.
Shells
- The maximum number of electrons that can be accommodated in a shell (n) is determined by the principal quantum number.
- The formula 2n2 is used to represent it, where 'n' is the shell number.
- The shells, n values, and the total number of electrons that can be accommodated are shown in the table below:
| Shell and ‘n’ value | Max. Electrons in the Electron Configuration |
|---|---|
| K shell, n=1 | 2 * 12 = 2 |
| L shell, n=2 | 2 * 22 = 8 |
| M shell, n=3 | 2 * 32 = 18 |
| N shell, n=4 | 2 * 42 = 32 |
Subshells
- The azimuthal quantum number (denoted by 'l') determines the subshells into which electrons are distributed.
- The value of this quantum number is determined by the value of the principal quantum number, n. As a result, when n equals 4, four different subshells are possible.
- When n = 4, The s, p, d, and f subshells correspond to l=0, l=1, l=2, and l=3 values, respectively.
- The formula 2*(2l + 1) gives the maximum number of electrons that a subshell can accommodate.
- As a result, the s, p, d, and f subshells can each hold a maximum of 2, 6, 10, and 14 electrons.
Electron Configuration Writing
The following table lists all of the possible subshells for n values up to 4:
| Principle Quantum No. Value | Value of Azimuthal Quantum No. | Subshell in the Electron Configuration |
|---|---|---|
| n=1 | I=0 | 1s |
| n=2 | I=0 | 2s |
| I=1 | 2p | |
| n=3 | I=0 | 3s |
| I=1 | 3p | |
| I=2 | 3d | |
| n=4 | I=0 | 4s |
| I=1 | 4p | |
| I=2 | 4d | |
| I=3 | 4f |
As a result, the 1p, 2d, and 3f orbitals do not exist because the value of the azimuthal quantum number is always less than the value of the principal quantum number.
Read More: Electronegativity chart
Notation
- Subshell labels are used to write down an atom's electron configuration.
- These labels include the shell number (given by the principal quantum number), the subshell name (given by the azimuthal quantum number), and the total number of electrons in the subshell in superscript.
- For instance, if two electrons are filled in the first shell's 's' subshell, the resulting notation is '1s2'.
- With the help of these subshell labels, the electron configuration of magnesium (atomic number 12) can be written as 1s2 2s2 2p6 3s2.
Notation Writing
Read More: Long form of Periodic Table
Filling of Atomic Orbitals
[Click Here for Sample Questions]
The electrons in the atomic orbitals are filled up according to the following principles.
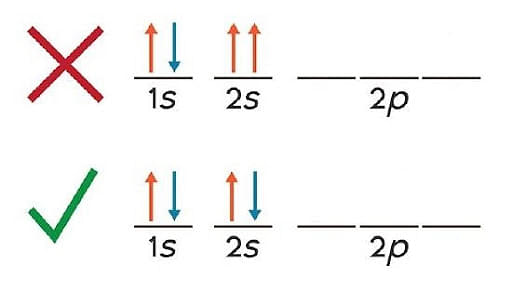
Aufbau Principle
- This principle is named after the German word 'Aufbeen,' which means 'to build up.'
- The Aufbau principle states that electrons will occupy lower energy orbitals before moving on to higher energy orbitals.
- The energy of an orbital is calculated by adding the principal and azimuthal quantum numbers.
- According to this principle, electrons are filled in the following order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p...
Aufbau principle illustrates the order in which electrons are filled in atomic orbitals:

Aufbau Principle
Note: It is important to note that the Aufbau principle has many exceptions, such as chromium and copper. The stability provided by half-filled or completely filled subshells can sometimes explain these exceptions.
Read more: Electron Gain Enthalpy
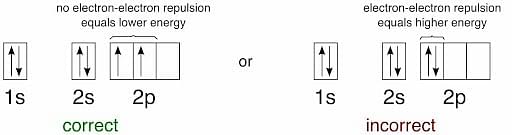
Pauli Exclusion Principle
- An orbital can only hold two electrons with opposite spins, according to the Pauli exclusion principle.
- This principle can be stated another way: "no two electrons in the same atom have the same values for all four quantum numbers."
- As a result, if two electrons have the same principle, azimuthal, and magnetic numbers, they must have opposite spins.

Pauli’s Exclusion Principle
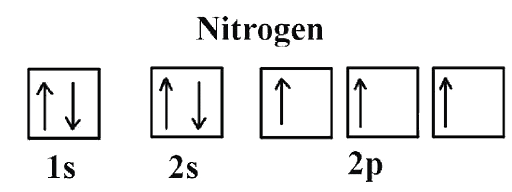
Hund's Rule
- Hund’s rule specifies the order in which electrons are filled in all subshell orbitals.
- Hund’s rule states that before a second electron is filled in an orbital, every orbital in a given subshell is singly occupied by electrons.
- To maximize the total spin, the electrons in orbitals with only one electron all have the same spin (or the same values of the spin quantum number).
Hund’s Rule
Read More: Oxidation States of Elements
Uses of Electron Configuration
[Click Here for Previous Year Questions]
Electron configurations can be used for a variety of, including:
- Determining an element's valency.
- Predicting a group of elements' properties (elements with similar electron configurations tend to exhibit similar properties).
- The interpretation of atomic spectra.
- This notation for the distribution of electrons in atomic orbitals came into use shortly after Ernest Rutherford and Niels Bohr presented the Bohr model of the atom in 1913.
Read More: Moseley’s Law
Electronic Configuration Examples
[Click Here for Sample Questions]
In this subsection, the electron configurations of a few elements are illustrated.
Hydrogen Electron Configuration
Hydrogen has an atomic number of one. As a result, a hydrogen atom contains one electron, which is assigned to the s subshell of the first shell/orbit. Hydrogen's electron configuration is 1s1, as shown below:
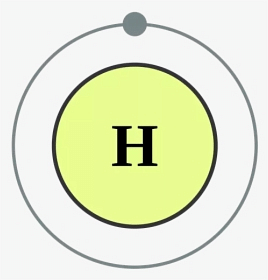
Hydrogen Electron Configuration
Also read: Ionisation Enthalpy
Electron Configuration of Oxygen
The atomic number of oxygen is 8, which means that each oxygen atom contains 8 electrons. The electrons are filled in the following order:
- K shell– 2 electrons
- L shell– 6 electrons
Therefore, the electron configuration of oxygen is 1s2 2s2 2p4 as shown below:
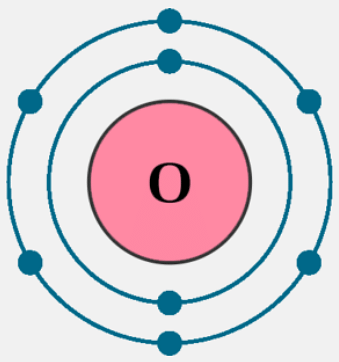
Oxygen Electron Configuration
Electron Configuration of Magnesium
Magnesium has an atomic number of 12. Therefore, its 12 electrons are distributed in the following manner:
- K shell– 2 electrons
- L shell– 8 electrons
- M shell– 2 electrons
The electron configuration of magnesium is illustrated below. The EC can be written as 1s2 2s2 2p6 3s22
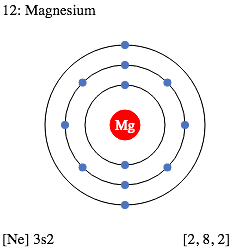
Magnesium Electron Configuration
Electronic Configuration of First 20 Elements
[Click Here for Previous Year Questions]
Tabulated below is the electronic configuration of the first 20 elements of the periodic table:
| Atomic Number | Name of Elements | Electronic Configuration |
|---|---|---|
| 1 | Hydrogen | 1s1 |
| 2 | Helium | 1s2 |
| 3 | Lithium | 1s2 2s1 |
| 4 | Beryllium | 1s2 2s2 |
| 5 | Boron | 1s2 2s2 2p1 |
| 6 | Carbon | 1s2 2s2 2p2 |
| 7 | Nitrogen | 1s2 2s2 2p3 |
| 8 | Oxygen | 1s2 2s2 2p4 |
| 9 | Fluorine | 1s2 2s2 2p5 |
| 10 | Neon | 1s2 2s2 2p6 |
| 11 | Sodium | 1s2 2s2 2p6 3s1 |
| 12 | Magnesium | 1s2 2s2 2p6 3s2 |
| 13 | Aluminum | 1s2 2s2 2p6 3s2 3p1 |
| 14 | Silicon | 1s2 2s2 2p6 3s2 3p2 |
| 15 | Phosphorus | 1s2 2s2 2p6 3s2 3p3 |
| 16 | Sulfur | 1s2 2s2 2p6 3s2 3p4 |
| 17 | Chlorine | 1s2 2s2 2p6 3s2 3p5 |
| 18 | Argon | 1s2 2s2 2p6 3s2 3p6 |
| 19 | Potassium | 1s2 2s2 2p6 3s2 3p6 4s1 |
| 20 | Calcium | 1s2 2s2 2p6 3s2 3p6 4s1 |
Read More:
| Important Topics | ||
|---|---|---|
| Clemmensen Reduction Reaction | Williamson Ether Synthesis | Tollen’s Test |
| Ruthenium | Rubidium | Lutetium |
| Periodic Acid Formula | Technetium | Strontium |
| Seaborgium | Rutherfordium | Scandium |
Important Topics for JEE MainAs per JEE Main 2024 Session 1, important subtopics included in the electron configuration are as follows:
Some memory based important questions asked in JEE Main 2024 Session 1 include:
i. Both statements I and II are correct. ii. Both statements I and II are incorrect. iii. Statement I is correct and statement II is incorrect. iv. Statement I is incorrect and statement II is correct. |
Things to Remember
- The distribution of electrons in an atom's orbitals is referred to as its electronic configuration.
- The periodic table distinguishes four types of elements based on their electronic configurations. These are the elements of the s-block, p-block, d-block, and f-block.
- The maximum number of electrons that can be accommodated in a shell is determined by the principal quantum number (n).
- The azimuthal quantum number (denoted by 'l') determines the subshells into which electrons are distributed.
- The Aufbau principle states that electrons will occupy lower energy orbitals before moving on to higher energy orbitals.
- The Pauli exclusion principle states that an orbital can only hold a maximum of two electrons with opposite spins.
- Hund’s rule specifies the order in which electrons are filled in all subshell orbitals.
NCERT Solutions for: Classification of Elements & Periodicity Properties
Previous Years’ Questions (PYQs)
- Which of the following does not have valence electron in 3d-subshell? [BITSAT 2012]
- Electronic configuration of S2− ion is? [JIPMER 1998]
- Which of the following has magic number of protons and neutrons? [AP EAPCET 1997]
- The outer electronic structure of 3s23p5 is possessed by? [KCET]
- The number of unpaired electrons in a paramagnetic diatomic molecule of an element with atomic number 16 is? [NEET 2006]
- Which one of the following orders is not in accordance with the property stated against it?… [NEET 2006]
- The first ionisation potential (in eV) of Be and B, respectively are… [NEET 1998]
- Identify the wrong statement in the following… [NEET 2012]
- Among the elements Ca,Mg,P and Cl, the order of increasing atomic radii is… [NEET 2010]
- Among the following which one has the highest cation to anion size ratio?… [NEET 2010]
- Which one of the following ions will be smallest in size?… [NEET 1996]
- The decreasing order of basic character of K2O,BaO,CaO and MgO is… [JIPMER 2015]
- Which one of the following arrangements represents the correct order of electron gain enthalpy… [NEET 2005]
- One of the characteristic properties of non-metals is that they… [NEET 1993]
- Which electronic configuration of an element has abnormally high difference between second and third ionisation energy?… [NEET 1993]
Sample Questions
Ques. What exactly is an element's electron configuration? (2 marks)
Ans. An element's electronic configuration is a symbolic representation of how its atoms' electrons are distributed across different atomic orbitals. A standardized notation is used when writing electron configurations, in which the energy level and type of orbital are written first, followed by the number of electrons present in the orbital written in superscript. Carbon, for example, has the electronic configuration 1s2 2s2 2p2 (atomic number: 6).
Ques. What is 1s 2s 2p 3s 3p? (2 marks)
Ans. The numbers 1s 2s 2p 3s 3p represent electron orbital energy levels. The orbital energy levels are always in the following order: -1s 2s = 2p 3s = 3p = 3d 4s = 4p = 4d= 4f A degenerate orbital is one that has the same energy as another orbital.
Ques. What are the exceptions to electron configuration rules? (2 marks)
Ans. There are two major exceptions to electron configuration: chromium and copper. A completely full or half-full d sub-level is more stable than a partially filled d sublevel in these cases, so an electron from the 4s orbital is excited and rises to the 3d orbital.
Ques. Define Aufbau Principle. (2 marks)
Ans. The Aufbau principle states that electrons will occupy lower energy orbitals before moving on to higher energy orbitals. The energy of an orbital is calculated by adding the principal and azimuthal quantum numbers. Electrons are filled in the following order, according to this principle: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p...
Ques. Define Hund’s Rule. (2 marks)
Ans. Hund’s rule specifies the order in which electrons are filled in all subshell orbitals. It states that before a second electron is filled in an orbital, every orbital in a given subshell is singly occupied by electrons. To maximize the total spin, the electrons in orbitals with only one electron all have the same spin (or the same values of the spin quantum number).
Ques. Define Pauli Exclusion Principle. (2 marks)
Ans. According to the Pauli exclusion principle, an orbital can only hold a maximum of two electrons with opposite spins. This principle can be stated another way: "no two electrons in the same atom have the same values for all four quantum numbers." As a result, if two electrons have the same principle, azimuthal, and magnetic numbers, they must have opposite spins.
Ques. What is Chlorine's Electron Configuration and how do you write it? (2 marks)
Ans. Chlorine has an atomic number of 17, implying that a chlorine atom has 17 electrons. It fills its electrons in the following order: 2 electrons in the K shell 8 electrons in the L shell 7 electrons in the M shell As a result, chlorine’s electron configuration is 1s2 2s2 2p6 3s2 3p5.
Ques. How should the electron configuration for neon be written? (2 marks)
Ans. The electron configuration of neon is written as the first two electrons in the electron configuration for neon will be in the 1s orbital. Because the 1s orbital can only hold two electrons, the next two electrons for Ne are placed in the 2s orbital. The remaining six electrons will be allocated to the 2p orbital. As a result, the Ne electron configuration is 1s2 2s2 2p6.
Ques. Why is the configuration of electrons in elements important? (2 marks)
Ans. Electron configurations help determine an atom's valence electrons, which provides insight into its chemical behaviour. It also aids in the categorization of elements into various blocks (such as the s-block elements, the p-block elements, the d-block elements, and the f-block elements).
Ques. Write the electronic configuration of Cl- ion. Also, find the total number of unpaired electrons in its ground state. (2 marks)
Ans. The electronic configuration of Cl is 1s22s22p63s23p5 . Since Cl- has one electron extra. So its p subshell will be fully paired. Hence the electronic configuration for Cl- ion will be
1s22s22p63s23p6
So the total number of unpaired electrons is zero.
Ques. Write the electronic configurations Fe2+ and find the total number of unpaired electrons in its ground state. (2 marks)
Ans. Fe2+ The electronic configuration of Fe is 1s22s22p63s23p63d64s2 .
For Fe2+ 2 electrons are removed from the 4s orbital so the resultant configuration is :
1s22s22p63s23p63d6 . All orbitals are completely filled except the 3d orbitals. We can write the arrangement of the 3d orbitals as follows
![]()
As is clear, the total number of unpaired electrons = 4.
Ques. What subshells are possible in n=3 energy level? How many orbitals are possible at this level? (2 marks)
Ans. We know that the values of the quantum number l determine the number of subshells.
For n=3, l has the values: 0,1 and 2. So three subshells s, p and d are possible at n=3 energy level.
For n=3 energy level we have three subshells- s,p and d subshells. We know that the subshell has 1 orbital. p has 3 orbitals and d subshell has 5 orbitals. So the total number of orbitals at n=3 energy level is 9.
Ques. What are the three rules to be followed at the time of writing the electronic configuration of elements? (3 marks)
Ans. The three rules that must be followed while writing electronic configuration of elements are:
- The Aufbau principle: Before occupying an orbital associated with a higher energy level, electrons must completely fill the atomic orbitals of a given energy level. Electrons occupy orbitals in the increasing order of orbital energy level.
- Pauli’s exclusion principle: According to this principle, no two electrons can have equal values for all four quantum numbers. Consequently, each subshell of an orbital can accommodate a maximum of 2 electrons and both these electrons must have opposite spins.
- Hund’s rule of maximum multiplicity: All the subshells in an orbital have to be singly occupied before any subshell is doubly occupied. Moreover, the spin of all the electrons in the singly occupied subshells must be the same.
Ques. What will be the total number of electrons that can be filled in s, p, and d subshell? (3 marks)
Ans. The s subshell can have a maximum of 2 electrons as it has only 1 orbital. The p subshell has 3 orbitals. Each orbital can accommodate a maximum of 2 electrons. Therefore, the p subshell can accommodate a maximum of 6 electrons. The d subshell has 5 orbitals and thus can accommodate a maximum of 10 electrons.
Check Out:



Comments