MHT CET 2024 24 April Shift 1 question paper is available for download here. MHT CET 2024 question paper comprises 150 MCQs carrying a total weightage of 200 marks.
MHT CET 2024 24 April Shift 1 Question Paper for PCB is divided into three subjects- Physics, Chemistry and Biology. The Physics and Chemistry section consists of 50 questions (10 questions from Class 11 and 40 questions from Class 12th syllabus). Meanwhile, the Biology Question Paper consists of 100 questions (20 questions from Class 11th and 80 questions from Class 12th syllabus).
MHT CET 2024 24 April Shift 1 Question Paper PDF Download
| MHT CET 2024 24 April Shift 1 Question Paper 2024 with Answer Key | Check Solutions |
MHT CET 2024 24 April Shift 1 Questions with Solutions
Okay, I understand. I will extract the questions from the provided PDF and present them in the requested format, ensuring that the solutions are detailed and follow the stepwise approach demonstrated in the document. Let's begin!
Question 1:
The potential energy of a particle performing linear S.H.M is 0.1π²x² joules. If the mass of the particle is 20 g, find the frequency of S.H.M:
Question 2:
A star 'A' has radiant power equal to 3 times that of the Sun. The temperature of star 'A' is 6000 K and that of the Sun is 2000 K. What is the ratio of their radii?
Question 3:
The speed of a wave is 30 m/s. If the distance between 11 crests is 1 m, what is the frequency (in Hz)?
Question 4:
The fundamental frequency of a closed organ pipe of length 20 cm is equal to the second overtone of an organ pipe open at both ends. What is the length of the organ pipe open at both ends?
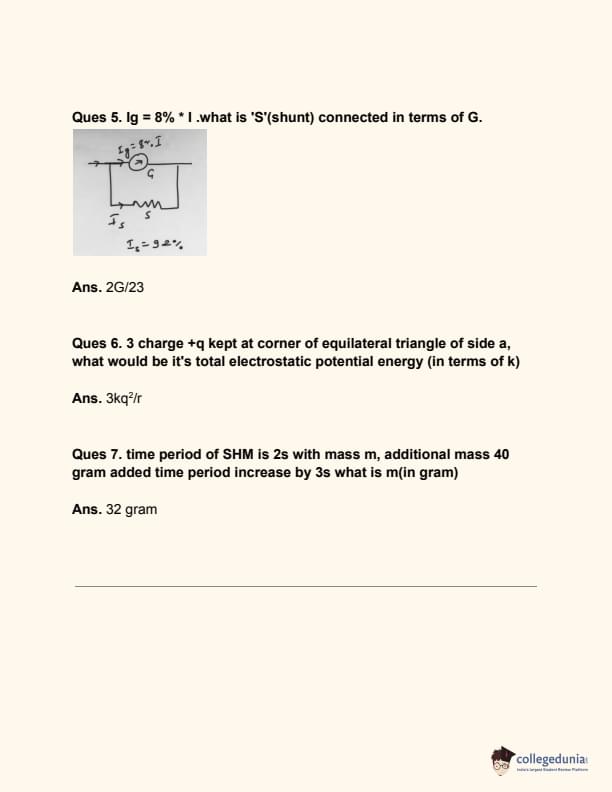
Question 5:
Ig = 8% × I. What is S (shunt) connected in terms of G?
Question 6:
Three charges +q are placed at the corners of an equilateral triangle of side a. What would be the total electrostatic potential energy (in terms of k)?
Question 7:
The time period of SHM is 2 s with mass m. If an additional mass of 40 g is added, the time period increases by 3 s. What is m (in grams)?
Question 8:
Who coined the term 'root pressure theory'?
Question 9:
How many of the following genotypes possibly represent normal wings in *Drosophila*?
(i) Vg⁺Vg⁺
(ii) Vg⁺Vgⁿⁱ
(iii) Vg⁺Vgⁿº
(iv) Vg⁺Vgˢᵗ
(v) Vg⁺Vg
Question 10:
Given below are two statements:
Statement I: Cell wall is freely permeable.
Statement II: Plasma membrane is selectively permeable.
Choose the correct answer from the options given below with reference to the structure of root hair:
Question 11:
Who discovered DNA?
Question 12:
Which of the following is not present in RNA?
Question 13:
Which of the following has a non-zero dipole moment?
Question 14:
How many moles of electrons are required for the reduction of 1 mole of Cr³⁺ to Cr⁰(s)?
Question 15:
What are the monomers of Bakelite?



Also Check:
MHT CET Previous Year Question Papers
| MHT CET 2023 Question Paper | MHT CET 2022 Question Paper |
| MHT CET 2021 Question Paper | MHT CET 2020 Question Paper |
| MHT CET 2019 Question Paper | MHT CET 2018 Question Paper |
Also Check:





Comments