Collegedunia Team Content Curator
Content Curator
Mean free path is defined as a gas molecule's average travel length between collisions. Every gas consists of an endless number of perfectly elastic spheres. When compared to the distance between them, the size of the gas molecules is insignificant. In a state of never-ending, fast, and random motion, the molecules of gas collide perfectly elastically with one another.
Also Check: Reversible and irreversible processes
| Table of Content |
Key Terms: Mean Free Path, Free Path, Kinetic Theory of Gas, Collisions, Gas Molecule, Ideal Gas, Cylinder, Velocity
Free Path and Mean Free Path
[Click Here for Sample Questions]
Free path between two successive collisions will be a straight path with unchanging velocity since the molecules exert no force on one another except when they collide. As a result, a single molecule's journey is made up of a succession of small zig-zag trajectories of varying lengths. These pathways of varying lengths are known as molecular free paths, and their mean is known as the mean free path.

Mean Free Path
In the kinetic theory of gases, we assume that the gas molecules are in constant motion, interacting with one another and with the container's walls. By its very nature, this form of collision is elastic.
Let's imagine there is 'n' number of molecules. Now concentrate on molecule A inside the gas, which is moving at a random rate and colliding with each other one by one. Molecule A collides with molecule B, then with C, and finally with D. As a result, it continues to collide with all molecules.
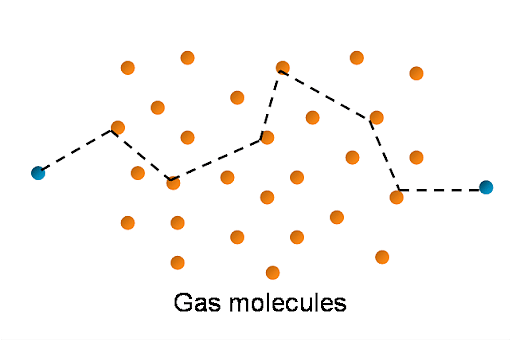
Motion of Gas Molecules
Consider the distance between the first and second collisions to be one, the distance between the second and third collisions to be two, and finally the distance between the third and fourth collisions to be three. 1, 2, and 3 are free pathways, meaning that the path between each collision is free.
So, at 1, molecule A got the first free path after colliding with molecule B, at 2, it got another free path after colliding with molecule C, and so on.
Because there are no collisions between any two collisions, the path between them is clear. As a result, the free path is defined as the distance between two consecutive collisions, such as 1, which is the free path. Likewise, 2 is a free path, and so on.
Frequently Asked Questions About Mean Free PathQues. What is considered the SI unit of temperature? (1 mark) Ans. Kelvin is the SI unit of temperature. What is considered the unit of molar mass? (1 mark) Ans. Kg mol−1 the unit of molar mass. |
What is Mean free path?
[Click Here for Previous Year's Questions]
Mean free path (λ) of a gas molecule can be defined as its average path length between collisions. Imagine the movement of a gas molecule inside an ideal gas. A typical molecule within an ideal gas would change its direction and speed abruptly as it collides elastically with other molecules of the same gas.
Though the molecule will move in a straight path at a constant speed between collisions, this is true for all the molecules in the gas. Mathematically, mean free path is given by,
| λ=\(\frac{1}{\sqrt{2} \pi d^2 \frac{N}{V}}\) |
We aim to quantify the mean free path since it is difficult to measure or characterize the random motion of gas molecules.
It varies inversely with N/V, which is the number of molecules per unit volume or the density of molecules because the more molecules there are, the greater the possibilities of them clashing with one another, thus reducing the mean free path \(\lambda\), and inversely proportional to the diameter d of the molecules because if the molecules were point masses, they would never collide.
Read More:
Derivation of Mean free path
[Click Here for Sample Questions]
We'll use the following assumptions to derive the equation:
- The molecule is spherical,
- The collision occurs when one molecule collides with another
- Only the molecule we're interested in is moving while the other molecules remain stationary
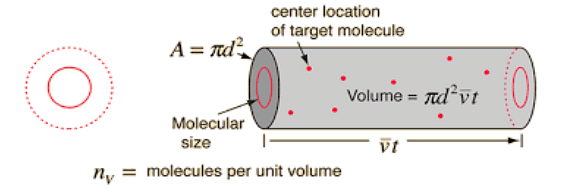
Mean Free path derivation
Assume that our solitary molecule has a diameter of d and that all other molecules are points. As our single-molecule passes through the gas, it sweeps out a short cylinder of a cross-section area d2, which does not affect our criteria for collision.
It will move vt between subsequent impacts for a little time t, where v is the molecule's velocity; now, if we sweep this cylinder, we will receive a volume of \(\pi d^2 \times vt\)
As a result, the number of point molecules in this volume will tell us how many collisions the molecule has. The number of molecules in the cylinder will equal N/V multiplied by the cylinder volume, i.e.,
| \(\pi d^2 \times vt\) |
λ = \(\frac{\text{length of the path during time(t)}}{\text{number of collisions in time(r)}} = \frac{\pi d^2vt}{\pi d^2 vt \frac{N}{V}}\)
→ \(\frac{1}{\pi d^2 \frac{N}{V}}\)
We have approximated the equation because we assumed that all the particles are stationary for the particle we are studying, when in fact, all the molecules are moving relative to each other. In the above equation, we have cancelled two velocities, but the v in the numerator is the average velocity and v in the denominator is relative velocity, so they both differ by a factor of \({\sqrt{2}}\).
As a result, the final equation is,
| λ = \(\frac{1}{{\sqrt{2}} \pi d^2 \frac{N}{V}}\) |
At sea level, the mean free path is 0.1 micrometres.
Read More: Period Motion
Previous Year Questions
- A 15 g mass of nitrogen gas is enclosed in a vessel at a temperature … [JEE Mains 2019]
- Two gases – argon (atomic radius 0.07nm, atomic weight 40) … [JEE Mains 2020]
- A vertical closed cylinder is separated into two parts by a frictionless … [JEE Mains 2019]
- An ideal gas occupies a volume of … [JEE Mains 2019]
- Consider two ideal diatomic gases … [JEE Mains 2020]
- If 1022 gas molecules each of mass 10−26 kg collide with a surface … [JEE Mains 2020]
- In a process, temperature and volume of one mole of an ideal monoatomic gas … [JEE Mains 2019]
- The approximate depth of an ocean is 2700m. The compressibility of water is…? [BITSAT 2015]
- A ball whose density is 0.4×103kg/m3 falls into the water from a height of 9cm. To what …? [BITSAT 2013]
- Water is supplied to different localities from a tank. The pressure of water is? [JIPMER 1996]
- A beaker of radius of 6 cm is filled with mercury up to a height of 12 cm... [AMUEEE 1998]
- A boy has 60kg weight. He wants to swim in a river with the help of a wooden log…? [MU OET 2011]
- A large drop of oil (density 0.8g/cm3 and viscosity η0 ) floats up through a column of…? [BITSAT 2012]
- In an ideal gas at temperature TT, the average force that a molecule applies … [JEE Mains 2015]
- An ideal gas is expanding such that … [JEE Advanced 2008]
- Cv and Cp denote the molar specific heat capacities of a gas at … [JEE Advanced 2009]
- Two spherical soap bubbles of diameters 10 cm and 6 cm are formed, one at each end of a… [KEAM]
Things to Remember
- Free path between two successive collisions will be a straight path with unchanging velocity since the molecules exert no force on one another except when they collide.
- A single molecule's journey is made up of a succession of small zig-zag trajectories of varying lengths. These pathways of varying lengths are known as molecular free paths, and their mean is known as the mean free path.
- The number of point molecules in this volume will tell us how many collisions the molecule has. The number of molecules in the cylinder will equal N/V multiplied by the cylinder volume, i.e., d2 x vt
- At sea level, the mean free path is 0.1 micrometres.
Read Also
Sample Questions
Ques. When Hydrogen is substituted by Helium, why does the mean free path increase? (2 marks)
Ans. In the case of the mean free path, as the gas density of molecules approaches each other, they are free to like each other, and as the volume decreases, the density of molecules grows, reducing the mean free path. As a result, when H2 is replaced by He, the mean free path grows.
Ques. When the pressure is increased, what happens to the mean free path? (2 marks)
Ans. With increased pressure, the mean free path shrinks. As the pressure rises, the volume shrinks, and the density rises. As molecules come closer to one another, the mean free path shrinks.
Ques. The molecular diameter of a gas in a 1 m 3 container is 0.1 m. There are ten molecules in all. Find the mean free path? (2 marks)
Ans. Mean free path is evaluated by the formula,
λ=\(\frac{1}{{\sqrt{2}} \pi d^2 N}\)
Where d is the diameter of the molecules and n is the number of molecules
\(\frac{1}{{\sqrt{2}} \pi \times 0.1 \times 0.1 \times 10}\)
= 2.25m
Ques. At 300 K and 1 atm, an oxygen molecule has a diameter of 1.2 1010m and travels through air at 300 K and 1 atm. Calculate the oxygen molecule's mean free path. (2 marks)
Ans. We know that,
λ=\(\frac{1}{{\sqrt{2}} \pi d^2 N}\)
We can find the number density n by using Ideal gas equation
n = \(\frac{N}{V}\)=\(\frac{P}{kT}\)= \(\frac{101.3 \times 10^3}{1.381 \times 10^{-23} \times 300}\)
= 2.449 X 1025 molecules/m3
λ=\(\frac{1}{{\sqrt{2}} \pi \times 2.449 \times 10^{25} \times (1.2 \times 10^-10)^2}\)
= \(\frac{1}{15.65 \times 10^5}\)
=0.63 X 10-6m
Ques. What is the link between a molecule diameter and a mean free path? (2 marks)
Ans. Non-existing. For each type of molecule, the diameter is constant. The mean free path, which is mostly determined by density, is the distance between collisions of molecules during their thermal motions.
Ques. How Do You Increase the Mean Free Path? (2 marks)
Ans. The mean free path is the average distance a molecule travels between collisions, so the longer the molecules are apart, the longer the free path will be. As the gas density rises, the molecules collide with one another, reducing the open path. To increase the mean free path, it's vital to keep the molecules apart.
The formula for the mean free path is:
\(\lambda = \frac{RT}{ {\sqrt{2}}\pi d^2 NAP}\)
Ques. What Is the Effect of Temperature on the Mean Free Path? (2 marks)
Ans. According to the kinetic theory of gases, when the temperature rises, molecules move faster; however, the distance between them, or the mean free path, remains constant, and only the probability of colliding reduces. As a result, we can argue that the mean free path is temperature independent.
Ques. What Does Collision Frequency Mean? (2 marks)
Ans. The collision frequency is the regular time interval in which molecular collisions occur on average. The letter Z stands for it. Its formula is as follows:
Z = 1/f = T
Ques. Which gases are present in the Sun? (2 marks)
Ans. The sun is composed of a fiery mixture of gases. Plasma is the form in which these gases exist. The sun is a gaseous body, not a solid object. It lacks the discernible limits that rocky worlds like Earth have. Instead, the sun is built up almost completely of hydrogen and helium layers. The sun's layers are measured by their fraction of the sun's overall radius, and these gases perform various tasks in each layer.
Ques. What happens when molecules clash at ultra-high temperatures? (2 marks)
Ans. Collisions between molecules in the molecular beam and ambient background gases in the chamber are almost non-existent under UHV circumstances due to the huge λ values. Any change in the features of the initial molecular beam distribution seen in the product distribution can be extrapolated to have resulted exclusively from a collision with the target surface in the absence of interaction with background gases. Creating and sustaining a UHV environment is rarely a simple task. It is necessary to use specially constructed UHV chambers with carefully selected pumping systems.
Ques. Which are the factors which control Mean Free Path? (2 marks)
Ans. There are a few factors which control the mean free path. They are:
- The radius of the molecule
- Density
- Total number of molecules
- Pressure
- Temperature etc.
Also Read:




Comments