Bihar Board Class 12 Chemistry Question Paper 2023 PDF (Code 118 Set – F) is available for download here. The Biology (Elective) exam was conducted on February 6, 2023 in the Evening Shift from 9:30 AM to 12:45 PM. The total marks for the theory paper are 100. Students reported the paper to be easy to moderate.
Bihar Board Class 12 Chemistry Question Paper 2023 (Code 118 Set – F) with Solutions
| Bihar Board Class 12 Chemistry Question Paper with Answer Key | Check Solutions |

Which of the following ions of transition elements is paramagnetic?
View Solution
Step 1: Recall the rule for paramagnetism.
A species is paramagnetic if it has one or more unpaired electrons; otherwise, it is diamagnetic.
Step 2: Write d–electron configurations.
Ag\(^{+}\) : \(4d^{10}\) (all paired) \(\Rightarrow\) diamagnetic.
Cu\(^{2+}\) : \(3d^{9}\) (one unpaired) \(\Rightarrow\) paramagnetic.
Zn\(^{2+}\) : \(3d^{10}\) (all paired) \(\Rightarrow\) diamagnetic.
Au\(^{+}\) : \(5d^{10}\) (all paired) \(\Rightarrow\) diamagnetic.
Step 3: Conclusion.
Only Cu\(^{2+}\) has an unpaired electron; hence it is paramagnetic.
Quick Tip: For first-row transition-metal \emph{cations}, check the \(d\) count: \(d^{0}\), \(d^{10}\) are diamagnetic; any other \(d^{n}\) usually indicates paramagnetism due to unpaired electrons.
Which of the following is called coinage metals?
View Solution
Step 1: Definition.
Coinage metals are the Group 11 elements historically used to mint coins due to their malleability, luster, and resistance to corrosion.
Step 2: Identify the group.
Group 11 elements are copper (Cu), silver (Ag), and gold (Au).
Step 3: Analyze options and conclude.
Options (A), (B), and (D) either include non–Group 11 metals or omit one of the coinage metals; only option (C) lists Cu, Ag, and Au together.
Quick Tip: Remember: Group 11 \(=\) coinage metals \(\rightarrow\) Cu, Ag, Au — prized for low reactivity and high malleability.
Who gave the first important theory of coordination compounds?
View Solution
Step 1: Historical background.
The first important theory of coordination compounds was given by Alfred Werner in 1893. He proposed the idea of primary valency (ionisable) and secondary valency (non-ionisable) to explain coordination complexes.
Step 2: Analysis of options.
(A) Slater: Known for Slater’s rules in atomic structure, not coordination compounds.
(B) Pauling: Contributed valence bond theory, but not the first to explain complexes.
(C) Werner: Correct — He introduced the first scientific theory of coordination compounds.
(D) Lewis: Proposed Lewis acid-base concept, unrelated here.
Step 3: Conclusion.
Therefore, the correct answer is (C) Werner.
Quick Tip: Werner is known as the father of coordination chemistry and won the 1913 Nobel Prize in Chemistry.
Which of the following is an example of double salt?
View Solution
Step 1: Understanding double salts.
Double salts are crystalline salts obtained by the combination of two different salts. They dissociate completely into simple ions when dissolved in water.
Step 2: Analysis of options.
(A) Bleaching powder: A compound used as disinfectant, not a double salt.
(B) K\(_4\)[Fe(CN)\(_6\)]: This is a coordination compound, not a double salt.
(C) Hypo (Na\(_2\)S\(_2\)O\(_3\)·5H\(_2\)O): A simple hydrated salt, not a double salt.
(D) Potash alum: Correct — Potash alum (K\(_2\)SO\(_4\)·Al\(_2\)(SO\(_4\))\(_3\)·24H\(_2\)O) is a well-known double salt.
Step 3: Conclusion.
Thus, the correct answer is (D) Potash alum.
Quick Tip: Remember: Double salts lose their identity in solution, while coordination compounds retain their complex form.
Which of the following is a bidentate ligand?
View Solution
Step 1: Understanding bidentate ligands.
Bidentate ligands contain two donor atoms which can attach to a central metal ion, forming two coordinate bonds simultaneously.
Step 2: Analysis of options.
(A) E.D.T.A: This is a hexadentate ligand (can donate six pairs of electrons), not just bidentate.
(B) Ethylene diamine: Correct — It has two \(-NH_2\) groups, each donating a lone pair, making it bidentate.
(C) Acetate ion: Monodentate, binds through one oxygen atom.
(D) Pyridine: Monodentate, binds through nitrogen only.
Step 3: Conclusion.
Thus, the correct answer is (B) Ethylene diamine.
Quick Tip: Bidentate ligands form more stable chelate complexes due to the chelate effect.
The oxidation number of Pt in [Pt(C\(_2\)H\(_4\))Cl\(_3\)]\(^-\) is
View Solution
Step 1: Assign charges to ligands.
- Ethylene (C\(_2\)H\(_4\)) is a neutral ligand.
- Chloride (Cl\(^-\)) has charge \(-1\). There are 3 chlorides, so total = \(-3\).
Step 2: Apply oxidation number rule.
Let the oxidation number of Pt be \(x\). The overall charge is \(-1\). \[ x + (0 \times 1) + (-1 \times 3) = -1 \] \[ x - 3 = -1 \quad \Rightarrow \quad x = +2 \]
Step 3: Conclusion.
The oxidation number of Pt in [Pt(C\(_2\)H\(_4\))Cl\(_3\)]\(^-\) is +2.
Quick Tip: Always remember: neutral ligands like H\(_2\)O, NH\(_3\), C\(_2\)H\(_4\) contribute zero charge.
Which of the following is a Vinyl halide?
View Solution
Step 1: Definition.
A vinyl halide is a compound in which the halogen atom is directly attached to a carbon atom of a double bond (C=C).
Step 2: Analysis of options.
(A) CH\(_2\)=CH–CHBrCH\(_3\): Here Br is attached to an alkyl group, not directly to the double bond. Wrong.
(B) CH\(_2\)=CH–Br: Correct, because Br is directly bonded to the carbon of the C=C double bond.
(C) CH\(_2\)=CH–CH\(_2\)CH\(_2\)Cl: This is an allyl halide, not vinyl.
(D) HC≡C–Br: This is a haloalkyne, not vinyl halide.
Step 3: Conclusion.
The correct answer is (B) CH\(_2\)=CH–Br.
Quick Tip: Vinyl halides have the general structure R–CH=CH–X, where X is a halogen.
Enzymes are:
View Solution
Step 1: Definition.
Enzymes are biological catalysts that accelerate biochemical reactions in living organisms.
Step 2: Nature of enzymes.
Almost all enzymes are proteins in nature (except some catalytic RNAs known as ribozymes). They are highly specific in action and function under mild conditions of temperature and pH.
Step 3: Analysis of options.
(A) Carbohydrates: These act mainly as energy sources, not as enzymes.
(B) Lipids: Structural and storage roles, not catalytic.
(C) Proteins: Correct — enzymes are mainly globular proteins.
(D) None of these: Incorrect, since enzymes are proteins.
Step 3: Conclusion.
Thus, enzymes are proteins.
Quick Tip: Remember: All enzymes are proteins, but not all proteins are enzymes.
Which of the following is ether?
View Solution
Step 1: Understanding ether.
An ether is an organic compound in which an oxygen atom is bonded to two alkyl or aryl groups (R–O–R’).
Step 2: Analysis of options.
(A) R–C=O: Represents carbonyl group (aldehydes or ketones), not ether.
(B) R–C=O: Same as above, carbonyl compound.
(C) R–O–R: Correct, this is the general structure of ethers.
(D) HO–C=O: Represents carboxylic acid group, not ether.
Step 3: Conclusion.
The correct answer is (C) R–O–R.
Quick Tip: Ethers are often referred to as “alkoxy alkanes” and are used as solvents due to their low reactivity.
Which of the following is obtained on heating aqueous solution of benzene diazonium chloride?
View Solution
Step 1: Reaction principle.
Benzene diazonium chloride (C\(_6\)H\(_5\)N\(_2\)Cl) when heated with water undergoes hydrolysis to give phenol, nitrogen gas, and hydrochloric acid.
Step 2: Reaction.
\[ C_6H_5N_2Cl + H_2O \xrightarrow{\Delta} C_6H_5OH + N_2 + HCl \]
Step 3: Analysis of options.
(A) Benzene: Not formed here.
(B) Benzyl alcohol: Incorrect product.
(C) Phenol: Correct, formed as the major product.
(D) Chlorobenzene: Obtained with CuCl (Sandmeyer’s reaction), not by hydrolysis.
Step 4: Conclusion.
Thus, the correct answer is (C) Phenol.
Quick Tip: Remember: Simple heating of diazonium salts with water gives phenols. With Cu salts, different substituted benzene derivatives are obtained.
Which of the following is obtained when formic acid is heated with conc. H\(_2\)SO\(_4\)?
View Solution
Step 1: Reaction principle.
Formic acid (HCOOH) on dehydration with concentrated sulfuric acid undergoes elimination of water to form carbon monoxide (CO).
Step 2: Reaction.
\[ HCOOH \xrightarrow{conc.\ H_2SO_4,\ \Delta} CO + H_2O \]
Step 3: Analysis of options.
(A) CO\(_2\): Produced when formic acid is heated alone, not with conc. H\(_2\)SO\(_4\).
(B) CH\(_3\)HSO\(_4\): Not formed in this reaction.
(C) Oxalic acid: Not formed here.
(D) CO: Correct, dehydration product.
Step 4: Conclusion.
The correct answer is (D) CO.
Quick Tip: Formic acid is unique among carboxylic acids because it readily decomposes to CO in presence of conc. H\(_2\)SO\(_4\).
The IUPAC name of \(\mathrm{CH_3-CH(OH)-COOH}\) is
View Solution
Step 1: Identify the parent chain.
The compound has three carbons with a carboxylic acid group \((-COOH)\) \(\Rightarrow\) parent acid is propanoic acid.
Step 2: Locate and name the substituent.
There is an \(-OH\) (hydroxy) substituent on the second carbon: \(\mathrm{CH_3\!-\!CH(OH)\!-\!COOH}\).
Step 3: Write the IUPAC name.
Position + substituent + parent: \(\boxed{2-hydroxypropanoic acid}\).
Step 4: Conclusion.
Hence, the correct option is (B).
Quick Tip: In carboxylic acids, numbering starts from the carboxyl carbon; then name substituents with their positions.
Which of the following is Tollen's reagent?
View Solution
Step 1: Definition.
Tollen's reagent is an ammoniacal silver(I) solution containing the \([\mathrm{Ag(NH_3)_2}]^+\) (diamminesilver(I)) complex, usually present as \([\mathrm{Ag(NH_3)_2}]\mathrm{OH}\).
Step 2: Role.
It oxidizes aldehydes to acids and gets reduced to metallic silver (silver mirror test).
Step 3: Eliminate wrong options.
(B) \(\mathrm{Cu(OH)_2}\) and (D) \(\mathrm{CuO}\) are used in Fehling’s/Benedict’s tests; (C) \(\mathrm{Ag_2O}\) is not the ammoniacal complex.
Step 4: Conclusion.
Therefore, \([\mathrm{Ag(NH_3)_2}]^+\) is Tollen’s reagent \(\Rightarrow\) (A).
Quick Tip: Remember: \([\mathrm{Ag(NH_3)_2}]^+\) (ammoniacal Ag\(^+\)) gives the silver mirror with aldehydes but not with most ketones.
Which of the following gives Cannizzaro's reaction?
View Solution
Step 1: Condition for Cannizzaro reaction.
It occurs with aldehydes that lack \(\alpha\)-hydrogen. Such aldehydes undergo self-oxidation and self-reduction in strong base to give a salt of a carboxylic acid and an alcohol.
Step 2: Check options for \(\alpha\)-H.
(A) \(\mathrm{CH_3CHO}\): has \(\alpha\)-H \(\Rightarrow\) gives aldol, not Cannizzaro.
(B) \(\mathrm{HCHO}\) (formaldehyde): has no \(\alpha\)-H \(\Rightarrow\) undergoes Cannizzaro.
(C) \(\mathrm{HCOOH}\): not an aldehyde (it is a carboxylic acid).
(D) \(\mathrm{CH_3COCH_3}\): a ketone; does not show Cannizzaro.
Step 3: Conclusion.
Thus, \(\mathrm{HCHO}\) is the correct choice \(\Rightarrow\) (B).
Quick Tip: Aldehydes without \(\alpha\)-H (e.g., formaldehyde, benzaldehyde) give Cannizzaro; those with \(\alpha\)-H prefer aldol condensation.
Which of the following carbohydrates is the most abundant in nature?
View Solution
Step 1: Concept.
Cellulose is the most abundant carbohydrate on Earth. It is the structural component of plant cell walls.
Step 2: Analysis of options.
(A) Glucose: It is very common but not the most abundant polymer in nature.
(B) Fructose: A fruit sugar, less abundant overall.
(C) Starch: A storage carbohydrate, but not as abundant as cellulose globally.
(D) Cellulose: Correct, makes up plant biomass and is the most abundant carbohydrate.
Step 3: Conclusion.
Thus, the correct answer is (D) Cellulose.
Quick Tip: Cellulose forms about 50% of plant biomass and is the most abundant organic compound on Earth.
Which of the following disaccharides is present in milk?
View Solution
Step 1: Definition.
Lactose is the disaccharide sugar naturally found in milk and dairy products. It is composed of glucose + galactose.
Step 2: Analysis of options.
(A) Sucrose: Common sugar, found in sugarcane and beetroot, not in milk.
(B) Lactose: Correct — main sugar in milk.
(C) Maltose: Present in germinating grains, not in milk.
(D) None of these: Incorrect.
Step 3: Conclusion.
Hence, the correct answer is (B) Lactose.
Quick Tip: Remember: Lactose intolerance occurs when the enzyme lactase (which digests lactose) is deficient.
Which of the following is the chemical name of Vitamin A?
View Solution
Step 1: Concept.
Vitamin A is a fat-soluble vitamin essential for vision, growth, and immune function. Its chemical name is Retinol.
Step 2: Analysis of options.
(A) Thiamine: This is Vitamin B1.
(B) Retinol: Correct, this is Vitamin A.
(C) Ascorbic acid: This is Vitamin C.
(D) Nicotinamide: This is Vitamin B3.
Step 3: Conclusion.
Therefore, the chemical name of Vitamin A is Retinol (B).
Quick Tip: Vitamin A (Retinol) deficiency causes night blindness and xerophthalmia.
Which of the following vitamins are soluble in water?
View Solution
Step 1: Concept.
Vitamins are classified into water-soluble and fat-soluble categories. Water-soluble vitamins include Vitamin B-complex and Vitamin C. Fat-soluble vitamins are A, D, E, and K.
Step 2: Analysis of options.
(A) A and B: Incorrect, Vitamin A is fat-soluble.
(B) C and D: Incorrect, Vitamin D is fat-soluble.
(C) B and C: Correct, both are water-soluble.
(D) A and D: Incorrect, both A and D are fat-soluble.
Step 3: Conclusion.
Thus, the correct answer is (C) B and C.
Quick Tip: Water-soluble vitamins (B and C) are not stored in the body in large amounts and must be supplied regularly through diet.
From which of the following is insulin secreted?
View Solution
Step 1: Concept.
Insulin is a hormone secreted by the beta cells of the Islets of Langerhans in the pancreas. It regulates glucose metabolism.
Step 2: Analysis of options.
(A) Thyroid: Secretes thyroxine, not insulin.
(B) Pancreas: Correct, insulin is secreted here.
(C) Adrenal body: Secretes adrenaline, not insulin.
(D) None of these: Incorrect.
Step 3: Conclusion.
Thus, insulin is secreted by the pancreas, so the correct answer is (B).
Quick Tip: Diabetes mellitus occurs due to lack of insulin secretion or improper utilization of insulin.
Which of the following diseases is caused due to the deficiency of Vitamin E?
View Solution
Step 1: Recall diseases caused by vitamin deficiencies.
- Vitamin B1 (Thiamine) deficiency → Beri-beri.
- Vitamin C (Ascorbic acid) deficiency → Scurvy.
- Vitamin E deficiency → Reproductive issues and anti-fertility problems.
Step 2: Analysis of options.
(A) Beri-beri: Due to Vitamin B1 deficiency, not Vitamin E.
(B) Scurvy: Due to Vitamin C deficiency, not Vitamin E.
(C) Anti-fertility: Correct, caused by Vitamin E deficiency.
(D) None of these: Incorrect.
Step 3: Conclusion.
Thus, the disease caused by Vitamin E deficiency is Anti-fertility.
Quick Tip: Vitamin E is called the “anti-sterility vitamin” and is essential for reproductive health.
Natural rubber is a polymer of which of the following?
View Solution
Step 1: Recall natural rubber structure.
Natural rubber is obtained from the latex of rubber trees. It is a polymer of isoprene units \(\big(\mathrm{CH_2=C(CH_3)-CH=CH_2}\big)\).
Step 2: Type of polymerization.
It is formed by 1,4-addition polymerization of isoprene, resulting in cis-1,4 polyisoprene.
Step 3: Analysis of options.
(A) Ethylene: Polymerizes to form polyethylene, not rubber.
(B) Benzene: Aromatic, not polymerized to rubber.
(C) Isoprene: Correct — forms natural rubber.
(D) None of these: Incorrect.
Step 4: Conclusion.
Thus, natural rubber is a polymer of isoprene.
Quick Tip: Natural rubber = cis-1,4 polyisoprene; synthetic rubber (like neoprene) is made from other monomers.
The IUPAC name of \(\mathrm{CH_3-C(Br)(CH_3)_2-CH_3}\) is:
View Solution
Step 1: Identify the parent chain.
The longest chain contains 3 carbons \(\Rightarrow\) propane is the parent chain.
Step 2: Locate substituents.
- A bromo group is attached at carbon 2.
- A methyl group is also attached at carbon 2.
Step 3: Apply IUPAC rules.
The name becomes \(\mathrm{2-bromo-2-methylpropane}\).
Step 4: Check incorrect options.
(A) Tertiary butyl chloride: Wrong halogen.
(B) Secondary butyl chloride: Incorrect structure.
(C) 2-bromo-2-methyl propane: Correct IUPAC name.
(D) 2,2-dimethyl-1-bromoethane: Incorrect naming.
Step 5: Conclusion.
Thus, the correct name is (C) 2-bromo-2-methyl propane.
Quick Tip: Always choose the longest carbon chain as the parent chain, then assign substituents with the lowest possible numbers.
Alkyl halides are used for the preparation of which of the following?
View Solution
Step 1: Known conversions of alkyl halides.
Alkyl halides are versatile: (i) Alkanes via Wurtz coupling (R–X + Na → R–R),
(ii) Alkenes via \(\beta\)-elimination (alc. KOH), and (iii) Alcohols via nucleophilic substitution (aq. KOH).
Step 2: Conclusion.
Since all three can be prepared from alkyl halides, the answer is (D) All of these.
Quick Tip: Remember: SN1/SN2 (→ alcohol), E1/E2 (→ alkene), and coupling (→ alkane) are the three classic pathways for R–X.
Which of the following compounds has zero dipole moment?
View Solution
Step 1: Use molecular geometry.
CCl\(_4\) is perfectly tetrahedral and all C–Cl bond dipoles cancel due to symmetry.
Step 2: Eliminate others.
CH\(_3\)Cl, CHCl\(_3\), and CH\(_2\)Cl\(_2\) are polar because the vector sum of their bond moments is non-zero.
Step 3: Conclusion.
Hence, CCl\(_4\) has zero dipole moment.
Quick Tip: Zero dipole moment often indicates a highly symmetric geometry (e.g., linear, trigonal planar, tetrahedral with identical substituents).
Chloroform on reduction with zinc and water gives
View Solution
Step 1: Nature of the reaction.
Zinc in water acts as a reducing system providing nascent hydrogen, which dehalogenates haloforms.
Step 2: Apply to chloroform.
CHCl\(_3\) undergoes stepwise replacement of Cl by H under reducing conditions, ultimately forming CH\(_4\).
Step 3: Conclusion.
Therefore, chloroform reduces to methane \(\Rightarrow\) (D).
Quick Tip: Strong reducing conditions tend to convert haloforms (CHX\(_3\)) all the way to the parent hydrocarbon CH\(_4\).
Which of the following will not give iodoform test?
View Solution
Step 1: Recall iodoform test.
The iodoform test is given by compounds having the group \(-COCH_3\) or \(-CHOHCH_3\). Examples: ethanol, ethanal, and isopropyl alcohol.
Step 2: Analyze options.
(A) Isopropyl alcohol: Has \(-CHOHCH_3\), gives test.
(B) Ethanol: On oxidation gives acetaldehyde (\(-COCH_3\)), gives test.
(C) Ethanal: Directly has \(-COCH_3\), gives test.
(D) Benzyl alcohol: Does not have the required group, so does not give iodoform test.
Step 3: Conclusion.
Thus, the correct answer is (D) Benzyl alcohol.
Quick Tip: Mnemonic: Only compounds with \(-COCH_3\) or \(-CHOHCH_3\) groups respond to the iodoform test.
Butan-2-ol is a:
View Solution
Step 1: Structure.
Butan-2-ol = CH\(_3\)–CHOH–CH\(_2\)–CH\(_3\). The –OH group is attached to the second carbon.
Step 2: Classification.
The carbon bearing the –OH group is bonded to two other carbons → it is a secondary alcohol.
Step 3: Conclusion.
Hence, Butan-2-ol is a secondary alcohol.
Quick Tip: Alcohol classification depends on the carbon attached to the –OH group: 1° (one C), 2° (two C), 3° (three C).
Which of the following is soluble in water?
View Solution
Step 1: Solubility rule.
Polar compounds with hydrogen bonding are highly soluble in water. Non-polar compounds are insoluble.
Step 2: Analyze options.
(A) CH\(_3\)OH: Polar, forms H-bonds → soluble.
(B) CHCl\(_3\): Weak polarity, limited solubility.
(C) CCl\(_4\): Non-polar, insoluble.
(D) CS\(_2\): Non-polar, insoluble.
Step 3: Conclusion.
The correct answer is (A) CH\(_3\)OH.
Quick Tip: Small alcohols (like methanol, ethanol) are soluble in water due to hydrogen bonding, unlike non-polar halogenated compounds.
The total number of crystal systems is:
View Solution
Step 1: Recall the crystal systems.
In crystallography, the seven crystal systems are: cubic, tetragonal, orthorhombic, monoclinic, triclinic, hexagonal, and rhombohedral (trigonal).
Step 2: Eliminate wrong options.
- 8: Incorrect (there are 8 Bravais lattices types in cubic only, not crystal systems).
- 6: Incorrect.
- 4: Incorrect.
Step 3: Conclusion.
Thus, there are 7 crystal systems.
Quick Tip: Remember: There are 7 crystal systems and 14 Bravais lattices.
Which of the following is a covalent solid?
View Solution
Step 1: Types of solids.
- Metallic solids: Iron, Copper.
- Ionic solids: Sodium chloride.
- Covalent (network) solids: Diamond, quartz.
Step 2: Analysis.
Diamond is a covalent solid because each carbon atom is covalently bonded to four other carbon atoms in a tetrahedral arrangement, forming a giant covalent lattice.
Step 3: Conclusion.
Thus, the correct answer is (B) Diamond.
Quick Tip: Covalent solids are hard and have very high melting points due to strong covalent bonds throughout the lattice.
The coordination number of a metal crystallizing in a hexagonal close packed (hcp) structure is:
View Solution
Step 1: Recall definition.
The coordination number is the number of nearest neighbours surrounding a particle in a crystal lattice.
Step 2: hcp structure.
In hexagonal close packing, each atom is surrounded by 12 equidistant atoms (6 in the same layer, 3 above, and 3 below).
Step 3: Conclusion.
Thus, the coordination number in hcp is 12.
Quick Tip: Both hcp and fcc (cubic close packing) have a coordination number of 12, which represents maximum packing efficiency.
Which of the following is not an ideal solution?
View Solution
Step 1: Recall ideal solution definition.
An ideal solution obeys Raoult’s law at all concentrations and temperatures, with no enthalpy or volume change on mixing.
Step 2: Analyze options.
(A) Benzene + Toluene: Nearly ideal, similar structure.
(B) Methyl alcohol + Ethyl alcohol: Nearly ideal, similar polarities.
(C) Chloroform + Acetone: Not ideal — due to strong hydrogen bonding between chloroform (–H) and acetone (C=O), leading to negative deviation.
(D) CCl\(_4\) + SiCl\(_4\): Non-polar molecules, nearly ideal.
Step 3: Conclusion.
Thus, the solution of Chloroform + Acetone is not ideal.
Quick Tip: Hydrogen bonding or strong intermolecular interactions cause deviations from ideality.
Which of the following can pass through semi-permeable membrane?
View Solution
Step 1: Concept of osmosis.
Semi-permeable membranes allow only solvent molecules (commonly water) to pass through, but not solute particles.
Step 2: Analyze options.
(A) Solvent molecules: Correct — these can move across the membrane.
(B) Solute molecules: Blocked.
(C) Complex ions: Too large to pass.
(D) Simple ions: Generally do not cross without special ion channels.
Step 3: Conclusion.
Thus, solvent molecules can pass through semi-permeable membranes.
Quick Tip: Osmosis is solvent flow through a semi-permeable membrane from dilute to concentrated solution.
If 18 g of glucose is dissolved in 1000 g of solvent, then the solution is said to be:
View Solution
Step 1: Calculate moles of solute.
Molecular mass of glucose = 180 g/mol.
Moles of glucose = \(\frac{18}{180} = 0.1\) mol.
Step 2: Define molality.
Molality = (moles of solute) / (mass of solvent in kg).
Here solvent = 1000 g = 1 kg.
\[ Molality = \frac{0.1}{1} = 0.1 \, molal \]
Step 3: Analyze options.
- 1 molar: Wrong (molarity depends on solution volume, not mass of solvent).
- 0.1 molal: Correct.
- 0.1 molar: Incorrect because molarity ≠ molality here.
- 0.5 molal: Wrong, calculation mismatch.
Step 4: Conclusion.
Thus, the solution is 0.1 molal.
Quick Tip: Molality depends only on solvent mass, while molarity depends on total solution volume and varies with temperature.
Which of the following will have highest boiling point?
View Solution
Step 1: Use colligative property (boiling point elevation).
Boiling point elevation \(\Delta T_b = i\,K_b\,m\) depends on van’t Hoff factor \(i\) (number of particles).
Step 2: Compare \(i\) for solutes.
Glucose, sucrose, urea are non-electrolytes \(\Rightarrow i \approx 1\).
NaCl dissociates into Na\(^+\)+Cl\(^-\) \(\Rightarrow i \approx 2\).
Step 3: Conclusion.
Greater \(i\) gives larger \(\Delta T_b\). Hence 1% NaCl(aq) has the highest boiling point.
Quick Tip: Electrolytes raise boiling point more than nonelectrolytes of the same molality due to a larger van’t Hoff factor.
Which of the following gives acetone on oxidation?
View Solution
Step 1: Recall oxidation rules.
Secondary alcohols oxidize to ketones. Primary alcohols/aldehydes oxidize to acids.
Step 2: Classify options.
(A) Acetaldehyde (aldehyde) \(\rightarrow\) acetic acid.
(B) Ethanol (primary alcohol) \(\rightarrow\) acetaldehyde/acetic acid.
(C) Isopropyl alcohol (secondary) \(\rightarrow\) acetone.
(D) Methanol (primary) \(\rightarrow\) formaldehyde/formic acid.
Step 3: Conclusion.
Therefore, CH\(_3\)CHOHCH\(_3\) gives acetone on oxidation.
Quick Tip: Primary \(\rightarrow\) aldehyde/acid, secondary \(\rightarrow\) ketone, tertiary \(\rightarrow\) no easy oxidation (under mild conditions).
The functional group of a secondary amine is
View Solution
Step 1: Definition.
A secondary amine has the general formula \(\mathrm{R{-}NH{-}R'}\) with two alkyl/aryl groups attached to nitrogen and one hydrogen.
Step 2: Analyze options.
(A) \(-\mathrm{NH}-\): Correct representation of secondary amine linkage.
(B) \(-\mathrm{NH_2}\): Primary amine group.
(C) \(\mathrm{NH_3}\): Ammonia, not an amine functional group in an organic chain.
(D) \(\mathrm{NH_3^+}\): Protonated ammonium, not the neutral secondary amine group.
Step 3: Conclusion.
Hence, the functional group for a secondary amine is \(-\mathrm{NH}-\).
Quick Tip: Primary: \(\mathrm{R{-}NH_2}\); Secondary: \(\mathrm{R{-}NH{-}R'}\); Tertiary: \(\mathrm{R_3N}\).
The IUPAC name of CH\(_3\)–CH(NH\(_2\))–CH\(_3\) is:
View Solution
Step 1: Identify the parent chain.
The longest carbon chain = 3 carbons \(\Rightarrow\) propane.
Step 2: Locate substituent.
The –NH\(_2\) group is attached to the 2nd carbon.
Step 3: Apply IUPAC rule.
Thus, the correct IUPAC name = Propan-2-amine.
Step 4: Check options.
- (A) Propyl amine: common name.
- (B) Isopropyl amine: common name, not IUPAC.
- (C) Propan-2-amine: Correct IUPAC.
- (D) Propan-1-amine: Wrong carbon position.
Quick Tip: In naming amines, always use lowest locant for –NH\(_2\) group and parent hydrocarbon chain.
Primary amine on reaction with Grignard reagent gives which of the following?
View Solution
Step 1: Recall reaction type.
Grignard reagents (RMgX) are strong bases and react with acidic hydrogens. Primary amines (RNH\(_2\)) contain –NH hydrogen.
Step 2: Reaction.
RNH\(_2\) + RMgX \(\rightarrow\) R–MgNH + RH.
Thus, an alkane (RH) is formed.
Step 3: Eliminate options.
- Higher amine: Not possible.
- Secondary amine: Not formed.
- None of these: Incorrect.
Step 4: Conclusion.
Thus, the correct product is an alkane.
Quick Tip: Grignard reagents liberate alkanes from any compound with acidic hydrogen (e.g., water, alcohols, amines).
Which of the following is the most basic?
View Solution
Step 1: Recall effect of alkyl groups.
Alkyl groups release electrons by +I effect, increasing electron density on N and hence its basicity.
Step 2: Compare.
- (A) Aniline (C\(_6\)H\(_5\)NH\(_2\)): Lone pair delocalized into ring, basicity decreases.
- (B) Dimethylamine: Strong +I effect, lone pair available, very basic.
- (C) Trimethylamine: Steric hindrance reduces basicity in aqueous solution.
- (D) Ammonia: Less basic than substituted alkyl amines.
Step 3: Conclusion.
Most basic among them is (CH\(_3\))\(_2\)NH.
Quick Tip: Basicity order in aqueous medium: Secondary amine > Primary amine > Tertiary amine > Ammonia.
Which of the following is Hinsberg reagent?
View Solution
Step 1: Recall Hinsberg test.
The Hinsberg test distinguishes between primary, secondary, and tertiary amines. The reagent used is p-toluene sulphonyl chloride (TsCl).
Step 2: Explanation.
- Primary amines form soluble sulphonamides.
- Secondary amines form insoluble sulphonamides.
- Tertiary amines do not react.
Step 3: Conclusion.
Thus, Hinsberg reagent is p-toluene sulphonyl chloride.
Quick Tip: Remember: Hinsberg reagent = p-TsCl, used to differentiate types of amines.
Which of the following is formed when acetamide reacts with Br\(_2\)/KOH?
View Solution
Step 1: Recall the reaction.
Acetamide reacts with Br\(_2\)/KOH in the Hofmann bromamide reaction.
Step 2: Reaction details.
R–CONH\(_2\) \(\xrightarrow{Br_2/KOH}\) R–NH\(_2\) + CO\(_2\).
Here, CH\(_3\)CONH\(_2\) → CH\(_3\)NH\(_2\).
Step 3: Conclusion.
The product is methyl amine.
Quick Tip: Hofmann bromamide reaction converts amides into primary amines with one carbon less.
Which of the following is deposited at cathode on electrolysis of aqueous NaCl solution?
View Solution
Step 1: Recall electrolysis process.
In aqueous NaCl electrolysis:
- At cathode: Water is reduced more easily than Na\(^+\), producing H\(_2\) gas.
- At anode: Cl\(^-\) is oxidized to Cl\(_2\).
Step 2: Analyze options.
(A) Chlorine: Produced at anode, not cathode.
(B) Sodium: Not deposited in aqueous solution due to high reduction potential.
(C) Sodium amalgam: Forms in mercury cathode electrolysis, not here.
(D) Hydrogen: Correct — released at cathode.
Step 3: Conclusion.
Therefore, hydrogen is deposited at cathode.
Quick Tip: In aqueous NaCl electrolysis: Cathode → H\(_2\), Anode → Cl\(_2\), Solution remains basic (NaOH formed).
Which of the following conducts electricity in a solution?
View Solution
Step 1: Key idea.
Electric conduction in solutions occurs via movement of ions. Substances that produce ions in solution are called electrolytes.
Step 2: Check options.
(A) Electrolytes: Dissociate to give ions \(\Rightarrow\) conduct.
(B) Non-electrolytes: Do not ionize \(\Rightarrow\) do not conduct.
(C) H\(_2\)O molecules: Pure water has extremely low conductivity.
(D) Copper wire: Conducts as a solid metal, not “in solution.”
Step 3: Conclusion.
Therefore, electrolytes conduct electricity in solution.
Quick Tip: Strong electrolytes (e.g., NaCl, HCl) conduct better than weak electrolytes (e.g., CH\(_3\)COOH) due to higher ionization.
The standard electrode potentials of metals A, B, C and D are \(-3.05\,\mathrm{V}\), \(-1.66\,\mathrm{V}\), \(-0.40\,\mathrm{V}\) and \(+0.8\,\mathrm{V}\) respectively. Which of the following would have the highest reducing power?
View Solution
Step 1: Recall rule.
More negative standard reduction potential (\(E^\circ\)) \(\Rightarrow\) stronger reducing agent (greater tendency to be oxidized).
Step 2: Compare values.
\(E^\circ(A)=-3.05\) V \(<\) \(E^\circ(B)=-1.66\) V \(<\) \(E^\circ(C)=-0.40\) V \(<\) \(E^\circ(D)=+0.8\) V.
Thus, A is the most negative and hence the strongest reducing agent.
Step 3: Conclusion.
Metal A has the highest reducing power.
Quick Tip: Remember: Most negative \(E^\circ\) = strongest \textbf{reducing} agent; most positive \(E^\circ\) = strongest \textbf{oxidizing} agent.
The cell constant of a conductivity cell is
View Solution
Step 1: Definition.
Cell constant (\(G^*\)) relates conductance to conductivity: \(\kappa = G^* \cdot G\), where \(G\) is conductance.
Step 2: Geometry dependence.
For parallel-plate electrodes, \(G^*=\dfrac{l}{A}\), with \(l\) = distance between electrodes and \(A\) = area of electrodes.
Step 3: Conclusion.
Hence, the cell constant is \(\dfrac{l}{A}\).
Quick Tip: Large electrode area or small spacing \(\Rightarrow\) smaller cell constant (more conductive cell).
The rate of the reaction \(2A + B \rightarrow C\) can be represented by which of the following?
View Solution
Step 1: Use stoichiometry in rate definition.
For \(aA+bB\rightarrow\) products, rate \(= -\dfrac{1}{a}\dfrac{d[A]}{dt} = -\dfrac{1}{b}\dfrac{d[B]}{dt} = +\dfrac{d[product]}{dt}\).
Step 2: Apply to \(2A + B \rightarrow C\).
Rate \(= -\dfrac{1}{2}\dfrac{d[A]}{dt} = -\dfrac{d[B]}{dt} = +\dfrac{d[C]}{dt}\).
Step 3: Conclusion.
All three expressions are valid \(\Rightarrow\) (D) All of these.
Quick Tip: Always divide the disappearance/appearance rate by the stoichiometric coefficient to get the unique rate.
The rate law of a reaction is \(Rate=k[A][B]^2\). If the concentration of \(B\) is trebled keeping \([A]\) constant, the rate becomes
View Solution
Step 1: Apply the rate law.
Initial rate \(r_1 = k[A][B]^2\). If \([B]\to 3[B]\) and \([A]\) constant, new rate
\(r_2 = k[A](3[B])^2 = 9\,k[A][B]^2 = 9r_1\).
Step 2: Conclusion.
Rate increases by a factor of 9 \(\Rightarrow\) nine times.
Quick Tip: If \(Rate\propto [X]^n\), multiplying \([X]\) by \(m\) multiplies the rate by \(m^n\).
The rate constant of a chemical reaction depends upon which of the following?
View Solution
Step 1: Arrhenius dependence.
The rate constant \(k\) varies with temperature as \(k = A e^{-E_a/RT}\). Hence \(k\) primarily depends on temperature.
Step 2: Eliminate others.
Mass, weight, and time do not directly determine the value of \(k\) (for a given reaction and medium).
Step 3: Conclusion.
Therefore, the correct answer is (A) Temperature.
Quick Tip: A rise of about \(10^\circ\)C often doubles the rate of many reactions due to the exponential \(k(T)\) dependence.
The important oxide ore of iron is
View Solution
Step 1: Identify iron ores.
Major iron ores: Haematite (Fe\(_2\)O\(_3\)), Magnetite (Fe\(_3\)O\(_4\)), Limonite, Siderite (FeCO\(_3\)).
Step 2: Choose oxide ore.
Among the options, Haematite is the oxide (Fe\(_2\)O\(_3\)) and a principal commercial ore of iron.
Step 3: Eliminate others.
Siderite is a carbonate; Pyrite is sulphide (FeS\(_2\)); Bauxite is an aluminium ore.
Step 4: Conclusion.
Hence, the correct answer is (B) Haematite.
Quick Tip: Remember: Haematite = Fe\(_2\)O\(_3\) (oxide), Siderite = FeCO\(_3\) (carbonate), Pyrite = FeS\(_2\) (sulphide).
In which of the following is the oxidation state of oxygen \(+2\)?
View Solution
Step 1: Assign oxidation states.
In \( \mathrm{F_2O} \) (oxygen difluoride), fluorine is more electronegative and has \(-1\) each. Let O be \(x\):
\( x + 2(-1) = 0 \Rightarrow x = +2 \).
Step 2: Eliminate others.
Cl\(_2\)O: O is \(-2\) (Cl is \(+1\)).
Na\(_2\)O\(_2\): Peroxide, O is \(-1\).
Na\(_2\)O: Oxide, O is \(-2\).
Step 3: Conclusion.
Only F\(_2\)O has oxygen in the \(+2\) state.
Quick Tip: Oxygen usually has \(-2\), but in peroxides it’s \(-1\), in superoxides \(-\tfrac{1}{2}\), and in oxygen difluoride it’s \(+2\).
Which of the following is a radioactive noble gas?
View Solution
Step 1: Fact check.
Among noble gases, radon (Rn) is naturally radioactive (formed in the decay chain of \(^{238}\)U and \(^{232}\)Th).
Step 2: Eliminate others.
He, Ne, and Xe are stable (non-radioactive) isotopes under normal conditions.
Step 3: Conclusion.
Therefore, the radioactive noble gas is Rn.
Quick Tip: Radon is the only naturally occurring radioactive noble gas commonly discussed at school level.
By which of the following noble gases have the maximum number of compounds been formed?
View Solution
Step 1: Known noble-gas compounds.
Xenon forms many stable compounds (e.g., XeF\(_2\), XeF\(_4\), XeF\(_6\), XeO\(_3\), XeO\(_4\)).
Step 2: Compare others.
He, Ne are extremely inert; Ar forms very few compounds (e.g., HArF at very low temperature).
Step 3: Conclusion.
Hence, the noble gas with maximum known compounds is Xenon.
Quick Tip: Heavier noble gases (Kr, Xe) are more polarizable and thus more reactive than He/Ne/Ar.
Which of the following is a polar compound?
View Solution
Step 1: Use shape to judge polarity.
SO\(_2\) is bent (V-shaped); its bond dipoles do not cancel \(\Rightarrow\) molecule is polar.
Step 2: Eliminate others.
SO\(_3\) (trigonal planar), BF\(_3\) (trigonal planar) and CO\(_2\) (linear) have symmetric geometries \(\Rightarrow\) overall non-polar.
Step 3: Conclusion.
Therefore, the polar compound here is SO\(_2\).
Quick Tip: Polarity depends on both bond polarity and \textbf{molecular geometry}; symmetric shapes often yield non-polar molecules.
Which of the following is a member of first transition series?
View Solution
Step 1: Define the first transition series.
The first transition series comprises the \(3d\) elements from Sc (21) to Zn (30).
Step 2: Classify options.
Ni (28) lies in the \(3d\) block \(\Rightarrow\) first transition series.
Ac (89) is an actinide; Au (79) belongs to the \(5d\) series; Cd (48) has a \(d^{10}\) configuration in its common oxidation state and is not considered a transition element.
Step 3: Conclusion.
Hence, Ni is the correct choice.
Quick Tip: First transition series = \(3d\) elements (Sc to Zn). Remember: Zn and Cd (with \(d^{10}\)) are not typical transition metals.
The electronic configuration of Cu\(^{2+}\) (Z = 29) is
View Solution
Step 1: Start from neutral Cu.
Cu atom: [Ar] \(3d^{10}4s^{1}\).
Step 2: Remove electrons for Cu\(^{2+}\).
Electrons are removed first from \(4s\) then \(3d\):
Cu\(^{2+}\): \([Ar]\,3d^{9}\).
Step 3: Conclusion.
Therefore, the configuration is [Ar] \(3d^{9}\).
Quick Tip: For transition metals, ionization removes \(ns\) electrons before \( (n-1)d \) electrons.
Which of the following contains ester linkages?
View Solution
Step 1: Identify polymer type.
Terylene (PET) is a polyester formed by condensation of terephthalic acid and ethylene glycol, containing repeated \(-\mathrm{COO}-\) (ester) linkages.
Step 2: Eliminate others.
Nylon: polyamide (\(-\mathrm{CONH}-\)) linkages.
Teflon: addition polymer of \(\mathrm{CF_2{=}CF_2}\), no ester.
Bakelite: phenol–formaldehyde resin (ether/methylene bridges), no ester.
Step 3: Conclusion.
Hence, Terylene contains ester linkages.
Quick Tip: Polyesters \(\Rightarrow\) \(-\mathrm{COO}-\) linkages; Polyamides (e.g., Nylon) \(\Rightarrow\) \(-\mathrm{CONH}-\).
Which of the following is a thermosetting plastic?
View Solution
Step 1: Define thermosetting plastics.
Thermosetting plastics are polymers that irreversibly harden when heated and cannot be remoulded. They form cross-linked structures.
Step 2: Analyze options.
- Nylon 6 and Nylon 6,6 are polyamides (thermoplastics).
- P.V.C. is a thermoplastic polymer.
- Bakelite is a phenol–formaldehyde resin, a thermosetting plastic.
Step 3: Conclusion.
Thus, the correct answer is Bakelite.
Quick Tip: Thermosetting plastics cannot be remoulded once set, unlike thermoplastics which soften on heating.
Which of the following is a polyamide?
View Solution
Step 1: Recall definition.
Polyamides are polymers containing \(-CONH-\) (amide) linkages.
Step 2: Analyze options.
- Teflon: poly(tetrafluoroethylene), no amide linkage.
- Nylon 6,6: condensation polymer of adipic acid and hexamethylenediamine → contains amide linkages.
- Terylene: polyester, contains ester linkages.
- Bakelite: phenol–formaldehyde resin, no amide linkage.
Step 3: Conclusion.
Hence, the correct answer is Nylon 6,6.
Quick Tip: Remember: Nylons are polyamides, while Terylene is a polyester.
Which of the following is used as an antacid?
View Solution
Step 1: Define antacids.
Antacids are substances that neutralize excess stomach acid (HCl).
Step 2: Analyze options.
- Magnesium hydroxide: a weak base used as an antacid.
- Phinacetin: analgesic drug (pain reliever), not an antacid.
- Penicillin: antibiotic.
- Sulphanilamide: antibacterial drug (sulfa drug).
Step 3: Conclusion.
Thus, the correct answer is Magnesium hydroxide.
Quick Tip: Common antacids include magnesium hydroxide, aluminum hydroxide, and sodium bicarbonate.
Saccharin is a/an
View Solution
Step 1: Definition.
Saccharin is an artificial sweetener, several hundred times sweeter than sucrose.
Step 2: Analysis of options.
- (A) Aliphatic hydrocarbon: Incorrect, saccharin is not a hydrocarbon.
- (B) Sweetening agent: Correct, saccharin is widely used as a non-nutritive sweetener.
- (C) Polynuclear compound: Incorrect, it is not polynuclear.
- (D) Sugar: Incorrect, saccharin is not a natural sugar but a synthetic compound.
Step 3: Conclusion.
Hence, the correct answer is Sweetening agent.
Quick Tip: Artificial sweeteners like saccharin and aspartame are used as sugar substitutes for diabetics.
Which of the following is used as a soap?
View Solution
Step 1: Recall soap definition.
Soaps are sodium or potassium salts of long-chain fatty acids.
Step 2: Check options.
- (A) C\(_{17}\)H\(_{35}\)COONa: Sodium stearate, a typical soap.
- (B) (C\(_{17}\)H\(_{35}\)COO)\(_2\)Ca: Insoluble salt (scum in hard water), not a soap.
- (C) C\(_{17}\)H\(_{35}\)COOH: Stearic acid, not soap.
- (D) C\(_{15}\)H\(_{31}\)COOH: Palmitic acid, also not soap.
Step 3: Conclusion.
Thus, the correct soap is C\(_{17}\)H\(_{35}\)COONa.
Quick Tip: Soaps are sodium/potassium salts, while calcium and magnesium salts form insoluble scum in hard water.
Which of the following is antioxidant?
View Solution
Step 1: Recall definition.
Antioxidants are substances that inhibit oxidation and protect the body from free radicals.
Step 2: Check options.
- (A) Lecithin: Acts as an antioxidant and emulsifier.
- (B) Citric acid: Works as a preservative and antioxidant.
- (C) Vitamin E: A natural antioxidant protecting cell membranes.
Step 3: Conclusion.
All the given compounds are antioxidants. Correct answer: All of these.
Quick Tip: Antioxidants delay spoilage of food and protect body tissues from oxidative stress.
The half life of a first order reaction
View Solution
Step 1: Formula of half-life.
For a first-order reaction, the half-life is given by: \[ t_{1/2} = \frac{0.693}{k} \]
Step 2: Dependence.
From the formula, we see that the half-life depends only on the rate constant \(k\), not on the initial concentration of the reactant.
Step 3: Analyze options.
(A) Incorrect, it actually depends on \(k\).
(B) Correct, it does not depend on initial concentration.
(C) Wrong, half-life is independent of initial concentration.
(D) Wrong, since only (B) is correct.
Step 4: Conclusion.
Hence, the correct answer is (B) does not depend upon initial concentration.
Quick Tip: For first-order reactions, half-life is constant and independent of initial concentration.
Which of the following catalysts is used in the manufacture of NH\(_3\) by Haber’s process?
View Solution
Step 1: Recall Haber’s process.
Ammonia is manufactured by Haber’s process: \[ N_2 + 3H_2 \leftrightharpoons 2NH_3 \quad (\Delta H = -92\,kJ) \]
Step 2: Catalyst used.
The catalyst used is finely divided iron with molybdenum as a promoter.
Step 3: Analyze options.
- Iron: Correct main catalyst.
- Molybdenum: Used only as promoter, not the main catalyst.
- Nickel: Used in hydrogenation, not Haber’s process.
- Platinum: Expensive and not used here.
Step 4: Conclusion.
Thus, the correct answer is (A) Finely divided iron.
Quick Tip: In Haber’s process: Catalyst = Finely divided iron, Promoter = Molybdenum.
By which of the following processes colloidal sols are purified?
View Solution
Step 1: Recall purification process.
Purification of colloidal sols involves the removal of impurities like electrolytes and small molecules.
Step 2: Analyze options.
- (A) Peptisation: Process of converting precipitate into colloidal sol, not purification.
- (B) Coagulation: Precipitation of colloids, not purification.
- (C) Dialysis: Correct, it removes soluble impurities by diffusion through a semipermeable membrane.
- (D) Flocculation: Causes aggregation of particles, not purification.
Step 3: Conclusion.
Thus, purification of colloidal sols is carried out by Dialysis.
Quick Tip: Dialysis and ultrafiltration are standard methods used for purification of colloidal solutions.
The highest volume of which of the following gases would be adsorbed by 1 g of charcoal at 298 K?
View Solution
Step 1: Recall principle of adsorption.
Extent of adsorption depends on the nature of the gas. More easily liquefiable gases are adsorbed more strongly.
Step 2: Compare given gases.
- H\(_2\): Least adsorbed, as it is highly non-polar and light.
- CH\(_4\): More adsorbed than H\(_2\), but weak van der Waals forces.
- CO\(_2\): More adsorbed due to higher polarity.
- NH\(_3\): Maximum adsorption, as it is highly polar and strongly interacting.
Step 3: Conclusion.
Thus, NH\(_3\) is adsorbed in the largest amount by charcoal.
Quick Tip: Greater polarity and ease of liquefaction of gas \(\Rightarrow\) greater adsorption on charcoal.
Pyrolusite is an ore of which of the following?
View Solution
Step 1: Recall definition.
Pyrolusite is the most common ore of manganese. Its formula is MnO\(_2\).
Step 2: Eliminate wrong options.
- Magnesium: ores are mainly dolomite and magnesite.
- Zinc: ores include zinc blende (ZnS) and calamine.
- Iron: ores include hematite, magnetite, siderite.
Step 3: Conclusion.
Thus, pyrolusite is an ore of Manganese.
Quick Tip: Pyrolusite (MnO\(_2\)) is used in dry cells and as a depolarizer.
The process of reduction of a metal oxide by carbon or carbon monoxide to the metal is called
View Solution
Step 1: Recall metallurgical process.
Smelting is the process in which a metal oxide is reduced to the free metal using carbon (coke) or carbon monoxide in a furnace.
Step 2: Analyze options.
- Roasting: heating sulfide ores in presence of oxygen, not reduction.
- Calcination: heating ores in absence of air, used to remove volatile impurities.
- Leaching: extraction using chemical solvents.
- Smelting: correct, involves reduction with carbon/CO.
Step 3: Conclusion.
Hence, the process is called Smelting.
Quick Tip: Smelting = reduction of ores with carbon/CO; Roasting = heating in air; Calcination = heating without air.
Cyanide process is used for the extraction of which of the following metals?
View Solution
Step 1: Recall cyanide process.
Cyanide process (MacArthur-Forrest process) is used for the extraction of gold (Au) and silver (Ag) from their low-grade ores.
Step 2: Check given options.
- Cr: not extracted by cyanide method.
- Ag: correct, silver is extracted by leaching with NaCN or KCN solution.
- Cu: not by cyanide process, usually by hydrometallurgy or smelting.
- Zn: extracted mainly by roasting and reduction.
Step 3: Conclusion.
Therefore, cyanide process is used for the extraction of Silver (Ag).
Quick Tip: Cyanide process is mainly applied to gold and silver ores.
What is molal elevation constant? How is it related to molarity of a solution?
View Solution
Step 1: Definition.
Molal elevation constant (also called ebullioscopic constant, \(K_b\)) is defined as the elevation in boiling point of a solvent when 1 mole of a non-volatile solute is dissolved in 1 kg of the solvent.
\[ \Delta T_b = K_b \cdot m \]
where, \(\Delta T_b\) = elevation in boiling point,
\(m\) = molality of the solution.
Step 2: Explanation.
It depends only on the nature of the solvent, not on the solute. The constant \(K_b\) has units of K·kg·mol\(^{-1}\).
Step 3: Relation with molality.
Since the formula involves molality, the rise in boiling point of a solution is directly proportional to its molality.
\[ \Delta T_b \propto m \]
Step 4: Distinction from molarity.
- Molality is defined per kg of solvent and is temperature-independent.
- Molarity is defined per liter of solution and is temperature-dependent.
Thus, molal elevation constant is connected with molality of the solution, not with molarity.
Quick Tip: Molal elevation constant (\(K_b\)) and molal depression constant (\(K_f\)) are examples of colligative property constants.
What do you understand by abnormal molecular mass?
View Solution
Step 1: Normal molecular mass.
Molecular mass of a solute is generally calculated from colligative properties (elevation of boiling point, depression of freezing point, osmotic pressure, relative lowering of vapor pressure). Ideally, this gives the correct molecular mass.
Step 2: Abnormal case.
Sometimes, the molecular mass determined experimentally using colligative properties differs from the theoretical (expected) value. This is called abnormal molecular mass.
Step 3: Causes of abnormal molecular mass.
- Association of molecules: Solute molecules combine to form aggregates (e.g., acetic acid in benzene). This leads to a molecular mass higher than expected.
- Dissociation of molecules: Solute molecules dissociate into ions (e.g., electrolytes like KCl in water). This leads to a molecular mass lower than expected.
Step 4: Explanation using van’t Hoff factor.
To correct this abnormal behavior, van’t Hoff introduced a factor \(i\): \[ i = \frac{Observed colligative property}{Calculated colligative property} \]
Then, modified formula: \[ \Delta T_b = i \cdot K_b \cdot m \] \[ \Delta T_f = i \cdot K_f \cdot m \]
Step 5: Conclusion.
Abnormal molecular mass occurs due to association or dissociation of solute molecules in solution. It is corrected using the van’t Hoff factor.
Quick Tip: If molecular mass is higher than normal → association; If molecular mass is lower than normal → dissociation.
What is peptization?
View Solution
Step 1: Definition.
Peptization is the process of converting a freshly precipitated substance (precipitate) into colloidal particles by adding a small amount of suitable electrolyte.
Step 2: Mechanism.
During peptization, the ions of the added electrolyte adsorb on the surface of precipitate particles. This develops an electric charge on the particles and prevents them from coagulating, leading to formation of a stable colloidal sol.
Step 3: Example.
When freshly prepared ferric hydroxide (\(Fe(OH)_3\)) precipitate is treated with a small amount of ferric chloride (\(FeCl_3\)) solution, a reddish brown colloidal sol of ferric hydroxide is formed.
\[ Fe(OH)_3 (ppt) + Fe^{3+} \rightarrow Fe(OH)_3 (sol) \]
Step 4: Importance.
Peptization helps in obtaining colloidal solutions from precipitates without complete dissolution.
Quick Tip: Peptization = conversion of precipitate → colloidal sol by electrolyte. The electrolyte ions stabilize the colloid.
What is sorption? Give an example.
View Solution
Step 1: Definition.
Sorption is a general term that refers to the simultaneous process of both adsorption and absorption taking place together.
Step 2: Explanation.
- Adsorption: The accumulation of molecules only on the surface of a solid or liquid.
- Absorption: The penetration of molecules into the bulk of a material.
When both these processes occur at the same time, the phenomenon is called sorption.
Step 3: Example.
Activated charcoal, when exposed to a dye solution, first adsorbs dye molecules on its surface and then some dye also diffuses inside its pores — this combined effect is called sorption.
Step 4: Real-life analogy.
Clothes dipped in water absorb and adsorb water molecules simultaneously — another example of sorption.
Quick Tip: Sorption = Adsorption + Absorption; it is common in porous solids and biological systems.
What is slag?
View Solution
Step 1: Definition.
Slag is the fusible (molten) waste material or by-product formed during the extraction of metals from their ores in a furnace. It mainly consists of impurities combined with flux.
Step 2: Formation.
In metallurgical processes, flux is added to combine with the gangue (earthy impurities like SiO\(_2\), Al\(_2\)O\(_3\)) to form slag. This slag is lighter and floats on the molten metal surface, protecting it from oxidation.
\[ Example: CaO + SiO_2 \rightarrow CaSiO_3 (slag) \]
Step 3: Function of slag.
- Removes impurities.
- Acts as a protective layer for the molten metal.
- Prevents oxidation.
Step 4: Example.
In the extraction of iron from hematite in a blast furnace, limestone (CaCO\(_3\)) acts as flux and reacts with silica impurities to form calcium silicate (slag).
Quick Tip: Slag = Flux + Gangue → waste by-product that floats over molten metal during smelting.
Nobel gases have comparatively large atomic radius. Why?
View Solution
Step 1: Nature of noble gases.
Noble gases (He, Ne, Ar, Kr, Xe, Rn) are monoatomic and chemically inert elements. They exist as isolated atoms rather than molecules.
Step 2: Mode of atomic radius measurement.
For noble gases, the atomic radius is determined as van der Waals radius, while for other elements, it is usually the covalent radius.
Step 3: Explanation.
The van der Waals radius is the distance between the nuclei of two non-bonded atoms in adjacent molecules. This distance is always larger than the covalent radius because the atoms are not bound tightly.
Step 4: Conclusion.
Hence, noble gases have comparatively large atomic radii because their atomic radius is measured as the van der Waals radius, not the covalent radius.
Quick Tip: Van der Waals radius > Covalent radius, therefore noble gases appear to have larger atomic sizes.
What are crystalline solids? Give example.
View Solution
Step 1: Definition.
Crystalline solids are those solids in which the constituent particles (atoms, ions, or molecules) are arranged in a definite, orderly, and repeating pattern in three-dimensional space.
Step 2: Characteristics.
- They have a definite geometric shape.
- Sharp melting point.
- Long-range order.
- Anisotropic in nature (properties vary in different directions).
Step 3: Examples.
Sodium chloride (NaCl), Diamond, Quartz, and Ice are examples of crystalline solids.
Step 4: Explanation.
In NaCl, the arrangement of Na\(^+\) and Cl\(^-\) ions is highly regular, forming a cubic lattice, which is a characteristic feature of crystalline solids.
Quick Tip: Crystalline solids show long-range order and definite geometry, unlike amorphous solids which have random arrangement.
What do you understand by point defects?
View Solution
Step 1: Definition.
Point defects are irregularities or deviations from the ideal arrangement of atoms or ions at specific lattice points in a crystalline solid.
Step 2: Explanation.
They occur due to the absence, displacement, or presence of extra atoms or ions at lattice sites. These defects affect the physical and electrical properties of the crystal.
Step 3: Types of point defects.
1. Stoichiometric defects – e.g., Schottky and Frenkel defects.
2. Non-stoichiometric defects – due to excess or deficiency of ions.
3. Impurity defects – caused by foreign atoms.
Step 4: Example.
In NaCl crystal, when equal numbers of Na\(^+\) and Cl\(^-\) ions are missing from their lattice sites, it produces a Schottky defect.
Quick Tip: Point defects disturb the arrangement at specific points; line defects disturb along an entire row of atoms.
What is formed when benzene reacts with ethyl bromide in the presence of anhydrous AlCl\(_3\)? Give equation.
View Solution
Step 1: Type of reaction.
This is an example of a Friedel–Crafts alkylation reaction. It involves the introduction of an alkyl group into an aromatic ring using an alkyl halide and a Lewis acid catalyst like anhydrous AlCl\(_3\).
Step 2: Reaction.
\[ C_6H_6 + C_2H_5Br \xrightarrow{AlCl_3} C_6H_5C_2H_5 + HBr \]
Step 3: Product formed.
The main product is ethylbenzene.
Step 4: Mechanism overview.
- AlCl\(_3\) reacts with ethyl bromide to generate the ethyl carbocation (C\(_2\)H\(_5^+\)).
- This carbocation then attacks the benzene ring, forming ethylbenzene after deprotonation.
Step 5: Importance.
This reaction is an important synthetic route for alkyl-substituted aromatic compounds.
Quick Tip: Friedel–Crafts alkylation = Benzene + Alkyl halide (in presence of AlCl\(_3\)) → Alkylbenzene.
What is power alcohol?
View Solution
Step 1: Definition.
Power alcohol is a mixture of ethyl alcohol (ethanol) and petrol (gasoline) used as a fuel for internal combustion engines.
Step 2: Composition.
Generally, power alcohol contains about 20–25% ethanol and 75–80% petrol, sometimes with small amounts of ether to improve performance.
Step 3: Purpose.
It is used to reduce dependence on petroleum fuels and to provide an alternative renewable energy source.
Step 4: Advantages.
- Increases octane number of fuel.
- Reduces environmental pollution.
- Utilizes renewable bio-based ethanol.
Step 5: Example.
A mixture of ethanol with petrol in proportions of 20:80 is used as motor fuel in several countries.
Quick Tip: Power alcohol = Ethanol + Petrol; used as a biofuel alternative in engines.
What are the important oxidation states of lanthanide elements?
View Solution
Step 1: General information.
Lanthanides are the 14 elements following lanthanum (Z = 57) from cerium (Ce) to lutetium (Lu). They belong to the f-block of the periodic table.
Step 2: Common oxidation state.
The most common oxidation state of lanthanides is +3, because after losing three electrons, they achieve a stable configuration with half-filled or fully filled f-subshells.
Step 3: Other oxidation states.
Some lanthanides also exhibit +2 and +4 oxidation states due to the stability of f\(^0\), f\(^7\), and f\(^{14}\) configurations.
Examples:
- Ce → +4 (Ce\(^{4+}\))
- Eu → +2 (Eu\(^{2+}\))
- Yb → +2 (Yb\(^{2+}\))
- Tb → +4 (Tb\(^{4+}\))
Step 4: Explanation.
This variable oxidation state arises due to the small energy difference between 4f, 5d, and 6s orbitals.
Quick Tip: Lanthanides commonly show +3 oxidation state; +2 and +4 states are also seen in a few cases due to f-orbital stability.
Explain the difference between a double salt and a complex salt.
View Solution
Step 1: Definition of Double Salt.
A double salt is formed by the combination of two simple salts in a definite proportion that crystallize together. In aqueous solution, it completely dissociates into its constituent ions.
Step 2: Definition of Complex Salt.
A complex salt consists of a central metal ion bonded to one or more ligands. It does not dissociate completely in water but gives a complex ion.
Step 3: Example.
- Double Salt: \( Mohr's salt (FeSO_4 \cdot (NH_4)_2SO_4 \cdot 6H_2O) \) dissociates completely into \( Fe^{2+}, NH_4^+, \) and \( SO_4^{2-} \).
- Complex Salt: \( K_4[Fe(CN)_6] \) does not dissociate completely; it gives complex ion \( [Fe(CN)_6]^{4-} \).
Step 4: Key Difference Table.
\begin{tabular{|p{4cm|p{5cm|
\hline
Double Salt & Complex Salt
\hline
Completely dissociates in water & Partially dissociates, forms complex ion
\hline
Exists only in solid state & Exists in solution as complex ions
\hline
Example: Mohr's salt & Example: Potassium ferrocyanide
\hline
\end{tabular
Quick Tip: Double salts dissociate completely; complex salts give stable complex ions in solution.
Give two examples of synthetic rubber.
View Solution
Step 1: Definition.
Synthetic rubbers are man-made polymers with elastic properties similar to natural rubber. They are obtained by polymerization of various monomers.
Step 2: Examples.
1. Buna-S (Styrene-butadiene rubber): Formed by copolymerization of 1,3-butadiene and styrene.
2. Neoprene: Formed by polymerization of chloroprene (2-chloro-1,3-butadiene).
Step 3: Uses.
- Buna-S is used in automobile tires.
- Neoprene is resistant to oil and chemicals and used for making hoses and gaskets.
Quick Tip: Synthetic rubbers are derived from petroleum-based monomers and designed for specific industrial applications.
What are preservatives?
View Solution
Step 1: Definition.
Preservatives are chemical substances added to food, drugs, cosmetics or other perishable materials to prevent spoilage caused by microbes (bacteria, fungi) or by chemical changes such as oxidation.
Step 2: How they work.
They either (i) inhibit microbial growth, (ii) kill microorganisms, or (iii) slow oxidation and rancidity.
Step 3: Common examples.
- Antimicrobial: sodium benzoate, benzoic acid, potassium metabisulphite (K\(_2\)S\(_2\)O\(_5\)), sodium nitrite (meats).
- Antioxidant: BHA (butylated hydroxyanisole), BHT (butylated hydroxytoluene), ascorbic acid.
Step 4: Conclusion.
Thus, preservatives extend shelf life and maintain safety/quality of products by preventing microbial and oxidative deterioration.
Quick Tip: Antimicrobials stop bugs; antioxidants stop oxygen. Read labels: E210 (benzoic acid), E300 (ascorbic acid), etc.
Arrange NH\(_3\), C\(_2\)H\(_5\)NH\(_2\), (C\(_2\)H\(_5\))\(_2\)NH and (C\(_2\)H\(_5\))\(_3\)N in the increasing order of their basic strength.
View Solution
Step 1: Principle.
In aqueous solution, basicity of amines depends on the electron-releasing (+I) effect of alkyl groups \emph{and solvation. General order:
\[ secondary > primary > tertiary > ammonia \]
because secondary amines have strong +I effect and still good solvation; tertiary amines suffer from steric hindrance and poorer solvation.
Step 2: Apply to given amines.
Least basic \(\to\) most basic: \[ \boxed{ \mathrm{NH_3 \;<\; (C_2H_5)_3N \;<\; C_2H_5NH_2 \;<\; (C_2H_5)_2NH } } \]
Step 3: Check.
This follows the aqueous-phase basicity trend: 2° > 1° > 3° > NH\(_3\).
Quick Tip: In water: 2° > 1° > 3° > NH\(_3\) (solvation matters). In gas phase, simple +I effect often gives 3° > 2° > 1°.
What is denaturation of proteins?
View Solution
Step 1: Definition.
Denaturation is the process in which a protein loses its native three-dimensional structure (secondary/tertiary/quaternary levels) due to disruption of non-covalent interactions (H-bonds, hydrophobic interactions, ionic bonds) and some S–S bridges, \emph{without breaking the peptide bonds of the primary structure.
Step 2: Causes.
Heat, extreme pH, heavy metal salts (Hg\(^{2+}\), Pb\(^{2+}\)), organic solvents (alcohols), urea/guanidinium salts, radiation, etc.
Step 3: Consequences.
Loss of biological activity (e.g., loss of enzyme function), loss of solubility, coagulation/precipitation.
Step 4: Examples.
- Coagulation of egg white on boiling.
- Curdling of milk with acid.
- High fever affecting enzyme activity.
Step 5: Note.
Renaturation is sometimes possible if the denaturing agent is removed gently and the primary structure remains intact.
Quick Tip: Denaturation ≠ hydrolysis: shape changes and activity is lost, but peptide backbone (primary structure) remains intact.
Give an example of a solution of a gas in solid.
View Solution
Step 1: Definition.
A solution of a gas in solid occurs when gas molecules are uniformly distributed throughout a solid medium. Such solutions are often called solid solutions of gases.
Step 2: Mechanism.
In these solutions, the gas particles occupy interstitial or void spaces within the crystal lattice of the solid without disturbing its structure.
Step 3: Example.
- Hydrogen in palladium (H in Pd) — Hydrogen gas gets adsorbed into palladium metal forming a solid solution.
This property is used in hydrogen storage and catalytic reactions.
Step 4: Explanation.
Palladium can absorb up to 900 times its volume of hydrogen due to the formation of a stable interstitial solid solution.
Quick Tip: Hydrogen in palladium is the classic example of a gas dissolved in a solid — used in hydrogen purification and catalytic systems.
What are isotonic solutions?
View Solution
Step 1: Definition.
Two or more solutions having the same osmotic pressure at a given temperature are called isotonic solutions.
Step 2: Explanation.
When two isotonic solutions are separated by a semipermeable membrane, there is no net movement of solvent molecules across the membrane, because both have equal osmotic pressure.
Step 3: Example.
- 0.9% NaCl solution (saline) is isotonic with human blood and tears.
Step 4: Relation.
Osmotic pressure (\(\pi\)) = \(CRT\), where \(C\) = concentration, \(R\) = gas constant, and \(T\) = temperature.
For isotonic solutions, \(\pi_1 = \pi_2\).
Quick Tip: Isotonic = equal osmotic pressure. Example: Medical saline (0.9% NaCl) and blood plasma.
What is soap? How does it act in cleansing clothes?
View Solution
Step 1: Definition.
Soap is the sodium or potassium salt of long-chain fatty acids (such as stearic, palmitic, or oleic acid).
General formula: C\(_{17}\)H\(_{35}\)COONa (sodium stearate).
Step 2: Mechanism of cleansing.
Soap molecules have two parts:
- A long non-polar hydrocarbon tail (hydrophobic) that repels water but attracts grease/oil.
- A polar carboxylate head (hydrophilic) that is attracted to water.
Step 3: Action.
When soap is added to water containing dirt or grease:
- The hydrophobic tail dissolves in grease.
- The hydrophilic head faces the water.
This forms spherical aggregates called micelles, which trap the oily dirt inside and wash away with water.
Step 4: Equation.
\[ Fat (Ester) + NaOH \xrightarrow{Boil} Soap + Glycerol \] Quick Tip: Soaps clean by forming micelles — oil in the center, hydrophilic ends outside — making grease water-washable.
How are metals refined by Bessemerisation?
View Solution
Step 1: Definition.
Bessemerisation is a metallurgical process used for the purification of metals, particularly for converting impure molten pig iron into steel or copper matte into pure metal.
Step 2: Principle.
In this process, air or oxygen is blown through molten metal, which oxidizes impurities such as carbon, silicon, sulfur, and iron into gaseous oxides or slag that can be removed.
Step 3: Reactions involved.
\[ 2C + O_2 \rightarrow 2CO, \quad Si + O_2 \rightarrow SiO_2, \quad Fe + O \rightarrow FeO \]
FeO then combines with silica to form slag (FeSiO\(_3\)), which floats and is removed.
Step 4: Application.
Used in the refining of copper and steel.
Step 5: Example.
In the Bessemer converter, impure blister copper is oxidized and converted into pure copper metal.
Quick Tip: Bessemerisation = blowing air through molten metal to oxidize and remove impurities.
What is the effect of temperature on reaction rate? What is Arrhenius equation?
View Solution
Step 1: Effect of temperature on reaction rate.
The rate of a chemical reaction generally increases with an increase in temperature. Usually, a rise of 10°C doubles or triples the rate of reaction.
Step 2: Reason.
When temperature increases, the kinetic energy of molecules also increases. This leads to more effective collisions between reactant molecules that have energy equal to or greater than the activation energy.
Step 3: Explanation using collision theory.
Only those collisions that occur with sufficient energy (≥ activation energy, \(E_a\)) and proper orientation result in product formation. Temperature increases the fraction of molecules possessing this minimum energy.
Step 4: Arrhenius Equation.
The temperature dependence of rate constant (\(k\)) is expressed by Arrhenius as: \[ k = A e^{-\frac{E_a}{RT}} \]
where, \(k =\) rate constant, \(A =\) frequency factor, \(E_a =\) activation energy, \(R =\) gas constant, \(T =\) absolute temperature (in K).
Step 5: Logarithmic form.
Taking logarithm on both sides: \[ \log k = \log A - \frac{E_a}{2.303R} \times \frac{1}{T} \]
This gives a straight line when \(\log k\) is plotted against \(\frac{1}{T}\) (Arrhenius plot).
Step 6: Conclusion.
Thus, the rate constant increases exponentially with temperature, leading to a faster reaction rate.
Quick Tip: Higher temperature → more molecules cross activation energy barrier → faster reaction rate. Arrhenius plot helps find \(E_a\).
What is electrochemical cell? Explain the structure of an electrochemical cell.
View Solution
Step 1: Definition.
An electrochemical cell is a device that converts chemical energy into electrical energy through redox reactions occurring in separate half-cells.
Step 2: Construction.
An electrochemical cell consists of:
- Two half-cells: one acts as the anode (oxidation) and the other as the cathode (reduction).
- A salt bridge that maintains electrical neutrality by allowing the flow of ions.
- Electrodes dipped into electrolyte solutions of their respective ions.
Step 3: Example — Daniell Cell.
\[ Zn | Zn^{2+} (aq) || Cu^{2+} (aq) | Cu \]
Here,
- At the anode: \( Zn \rightarrow Zn^{2+} + 2e^- \) (oxidation)
- At the cathode: \( Cu^{2+} + 2e^- \rightarrow Cu \) (reduction)
- Electrons flow from Zn to Cu through the external circuit.
Step 4: Working.
The redox reaction generates an electromotive force (emf) that drives the flow of electrons through the circuit.
Step 5: Cell notation and emf.
The overall reaction is: \[ Zn + Cu^{2+} \rightarrow Zn^{2+} + Cu \]
Cell emf is given by: \[ E_{cell} = E_{cathode} - E_{anode} \]
Step 6: Significance.
Electrochemical cells are used in batteries, corrosion studies, and electroplating.
Quick Tip: Remember: oxidation occurs at the anode, reduction at the cathode — “An Ox, Red Cat.”
(i) Explain the principle of manufacturing sulphuric acid by contact process.
View Solution
Step 1: Principle.
The manufacture of sulphuric acid by the Contact Process is based on the catalytic oxidation of sulphur dioxide (SO\(_2\)) to sulphur trioxide (SO\(_3\)) using vanadium(V) oxide (V\(_2\)O\(_5\)) as a catalyst.
Step 2: Chemical reactions involved.
\[ (a) Formation of SO_2: \quad S + O_2 \rightarrow SO_2 \] \[ (b) Oxidation of SO_2 to SO_3: \quad 2SO_2 + O_2 \xrightleftharpoons[V_2O_5]{450^\circC, 2 atm} 2SO_3 + Heat \]
Step 3: Conditions for maximum yield.
- Catalyst: V\(_2\)O\(_5\) (Vanadium(V) oxide)
- Temperature: around 450°C
- Pressure: about 1–2 atm
- The reaction is exothermic and reversible, hence low temperature and moderate pressure favor the forward reaction.
Step 4: Formation of sulphuric acid.
\[ SO_3 + H_2SO_4 \rightarrow H_2S_2O_7 \quad (oleum) \] \[ H_2S_2O_7 + H_2O \rightarrow 2H_2SO_4 \]
Step 5: Summary of process.
\[ S or FeS_2 \rightarrow SO_2 \xrightarrow{O_2, V_2O_5} SO_3 \xrightarrow{H_2SO_4} H_2S_2O_7 \xrightarrow{H_2O} H_2SO_4 \]
Step 6: Conclusion.
Thus, in the contact process, sulphuric acid is produced economically and efficiently through catalytic oxidation and absorption steps.
Quick Tip: Remember: Catalyst = V\(_2\)O\(_5\), Temperature ≈ 450°C, Pressure ≈ 2 atm. Always absorb SO\(_3\) in H\(_2\)SO\(_4\) (not directly in water).
(ii) Write the reaction of conc. sulphuric acid with oxalic acid.
View Solution
Step 1: Reaction involved.
When concentrated sulphuric acid reacts with oxalic acid (H\(_2\)C\(_2\)O\(_4\)), it acts as a dehydrating agent and also as an oxidizing agent.
Step 2: Chemical equation.
\[ H_2C_2O_4 \xrightarrow[Conc. H_2SO_4]{Heat} CO + CO_2 + H_2O \]
Step 3: Observation.
On heating, a mixture of carbon monoxide (CO) and carbon dioxide (CO\(_2\)) gases is evolved, and water vapor is released. The reaction mixture becomes warm due to its exothermic nature.
Step 4: Explanation.
Concentrated H\(_2\)SO\(_4\) removes water from oxalic acid and decomposes it into CO and CO\(_2\).
Quick Tip: Conc. H\(_2\)SO\(_4\) dehydrates oxalic acid to give CO and CO\(_2\) — a key test reaction for distinguishing H\(_2\)C\(_2\)O\(_4\).
“Compounds of transition elements are paramagnetic and coloured.” Explain.
View Solution
Step 1: Paramagnetic nature.
The compounds of transition elements show paramagnetism due to the presence of one or more unpaired electrons in their (n−1)d orbitals.
The magnetic moment (\(\mu\)) of such compounds is given by: \[ \mu = \sqrt{n(n+2)} Bohr Magneton (B.M.) \]
where \( n \) = number of unpaired electrons.
Step 2: Explanation.
- When an element or its ion has unpaired electrons, it is attracted by a magnetic field (paramagnetic).
- If all electrons are paired, the substance becomes diamagnetic.
Step 3: Examples.
- Fe\(^{2+}\): [Ar] 3d\(^6\) → 4 unpaired electrons → paramagnetic.
- Zn\(^{2+}\): [Ar] 3d\(^{10}\) → all paired → diamagnetic.
Step 4: Coloured nature.
Transition metal compounds are coloured due to the presence of incompletely filled d-orbitals.
When light falls on these compounds, electrons in the d-orbitals absorb specific wavelengths of light and jump from lower energy levels to higher ones (known as d–d transitions). The remaining transmitted light imparts colour.
Step 5: Examples.
- [Ti(H\(_2\)O)\(_6\)]\(^{3+}\) is purple due to d–d transition.
- [Cu(H\(_2\)O)\(_6\)]\(^{2+}\) is blue due to the absorption of red light.
Step 6: Conclusion.
Hence, the presence of unpaired d-electrons makes transition metal compounds both paramagnetic and coloured.
Quick Tip: Unpaired d-electrons = colour + magnetism. Full d\(^{10}\) configuration → colourless and diamagnetic.
How would you distinguish between the following compounds by chemical tests?
(i) Formaldehyde and Acetaldehyde
(ii) Acetaldehyde and Acetone
View Solution
(i) Formaldehyde and Acetaldehyde
Test: Schiff’s Reagent Test
- Formaldehyde gives a deep pink colour with Schiff’s reagent.
- Acetaldehyde gives only a light pink colour.
\[ HCHO + Schiff’s reagent \rightarrow Deep pink colour \]
(ii) Acetaldehyde and Acetone
Test: Iodoform Test
- Acetaldehyde (CH\(_3\)CHO) gives a yellow precipitate of iodoform (CHI\(_3\)).
- Acetone (CH\(_3\)COCH\(_3\)) also gives the iodoform test but formaldehyde does not.
\[ CH_3CHO + 3I_2 + 4NaOH \rightarrow CHI_3 + HCOONa + 3NaI + 3H_2O \]
Step 3: Observation.
Formation of yellow precipitate of CHI\(_3\) confirms the presence of a –COCH\(_3\) or –CHOCH\(_3\) group.
Quick Tip: Schiff’s reagent → detects formaldehyde. Iodoform test → confirms methyl ketone (–COCH\(_3\)) or ethanal.
“Formic acid behaves both as an aldehyde and an acid.” Explain.
View Solution
Step 1: Explanation.
Formic acid (HCOOH) is unique because it contains both the –CHO (aldehyde group) and the –COOH (carboxylic acid group). Hence, it exhibits the properties of both aldehydes and acids.
Step 2: Aldehydic nature.
Formic acid gives reactions typical of aldehydes such as:
- (i) Reducing nature: It reduces Tollen’s reagent and Fehling’s solution.
\[ HCOOH + 2[Ag(NH_3)_2]^+ + OH^- \rightarrow 2Ag + CO_2 + 3NH_3 + 2H_2O \]
Step 3: Acidic nature.
Formic acid also behaves as a typical carboxylic acid:
- It reacts with metals to liberate hydrogen gas.
\[ 2HCOOH + Zn \rightarrow (HCOO)_2Zn + H_2 \]
- It neutralizes alkalis to form formates.
Step 4: Conclusion.
Thus, due to the presence of both aldehyde and acid functional groups, formic acid exhibits dual behavior — reducing like an aldehyde and acidic like a carboxylic acid.
Quick Tip: Formic acid = HCOOH = only carboxylic acid with aldehydic character. Reduces Tollen’s reagent just like HCHO.





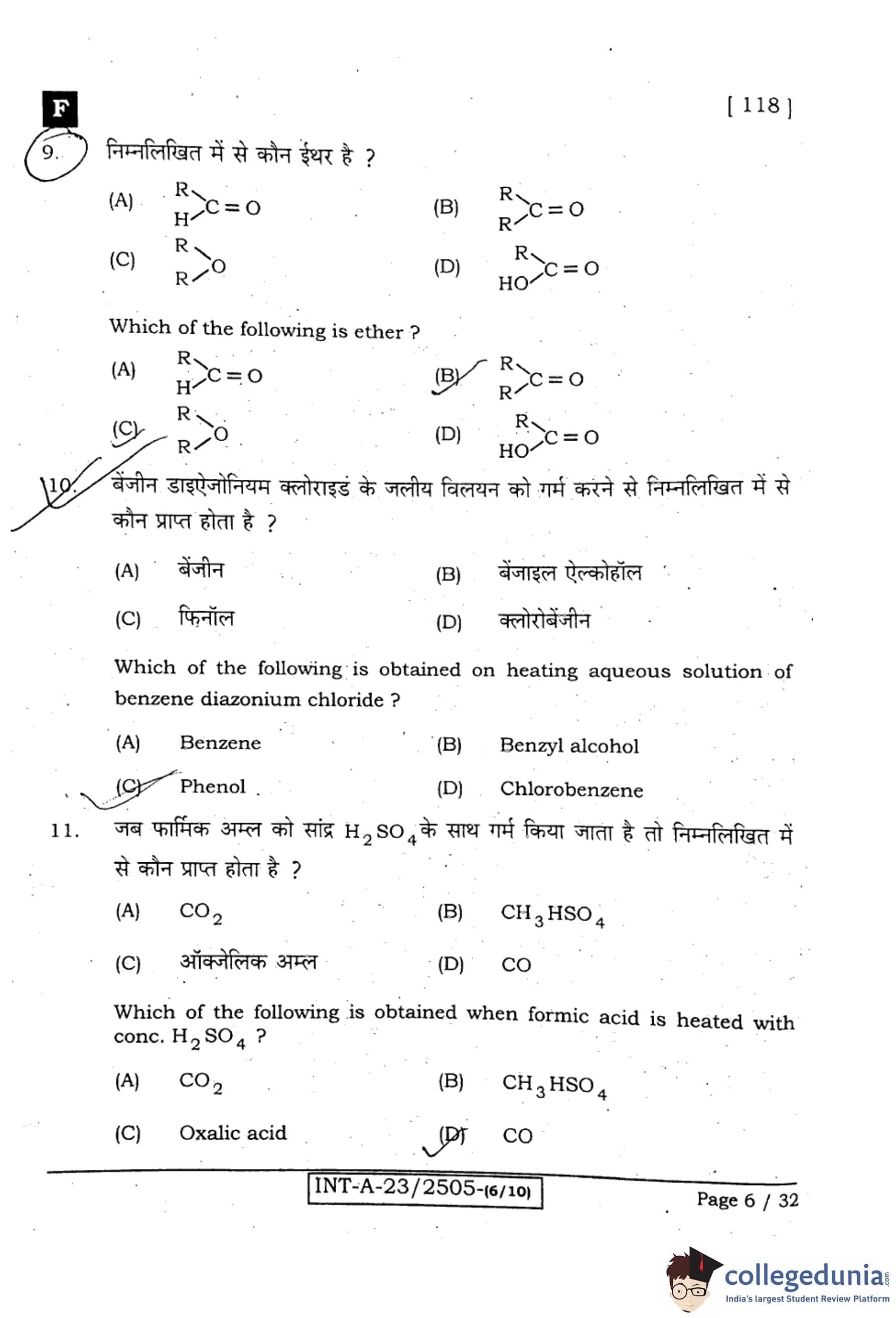




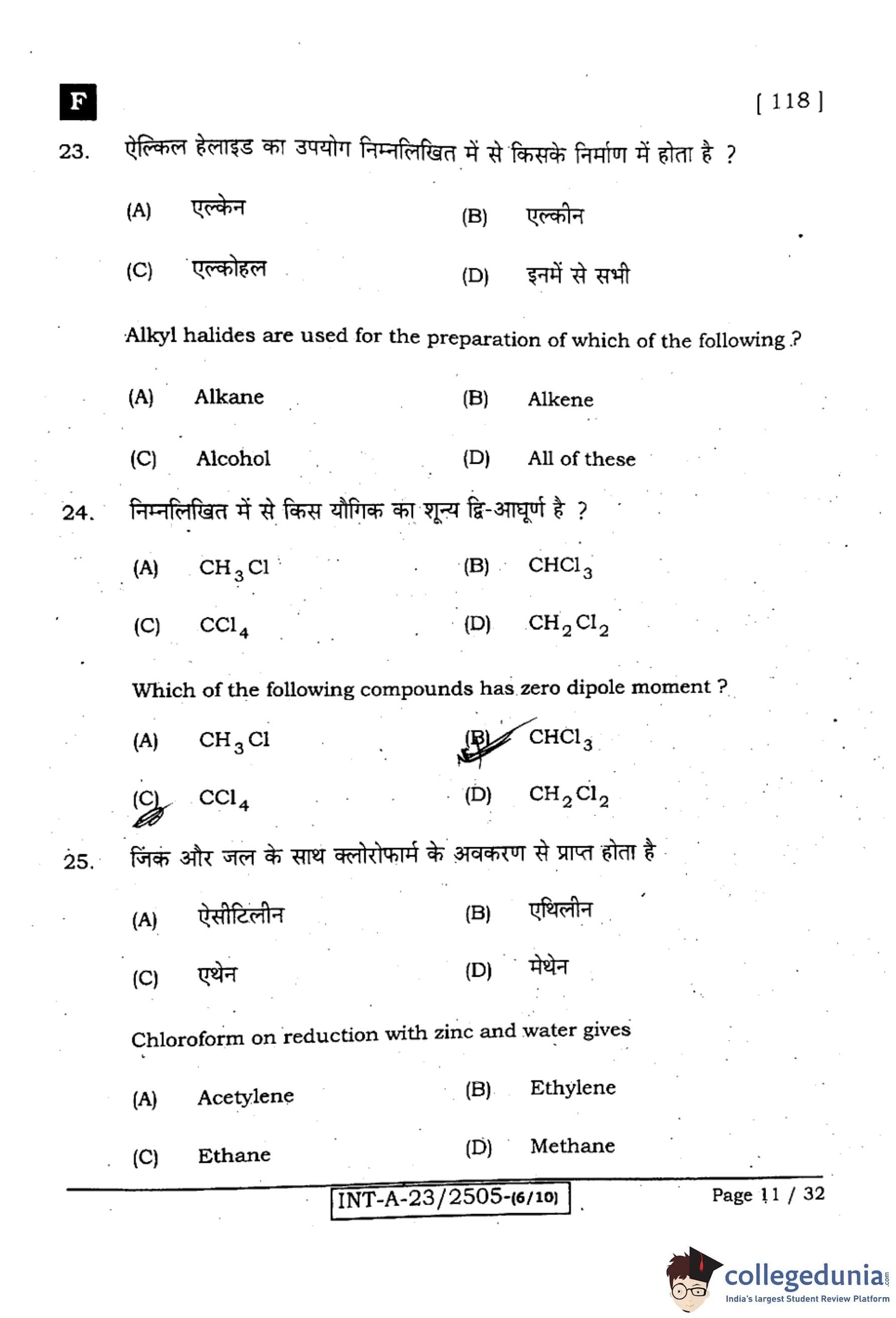

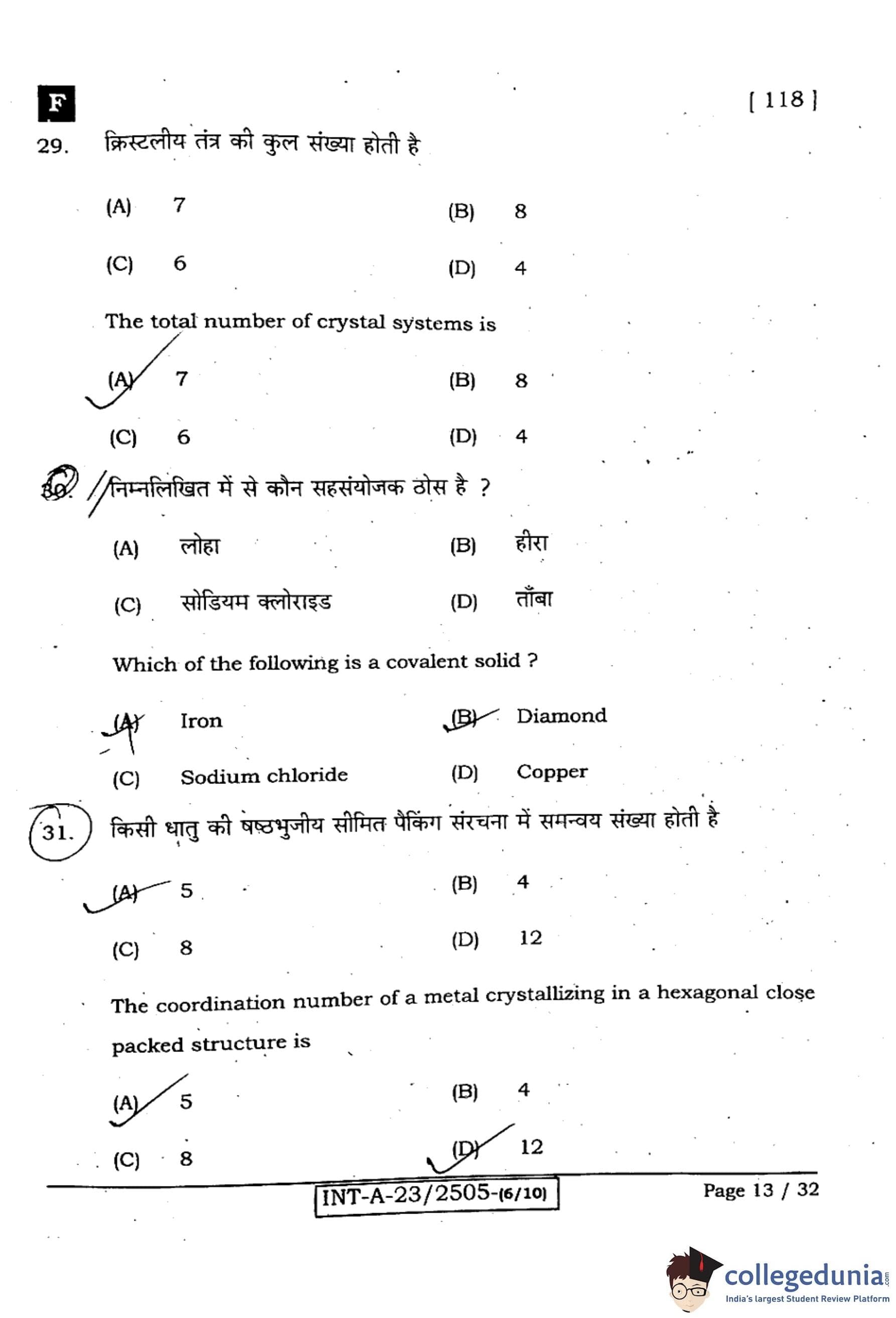





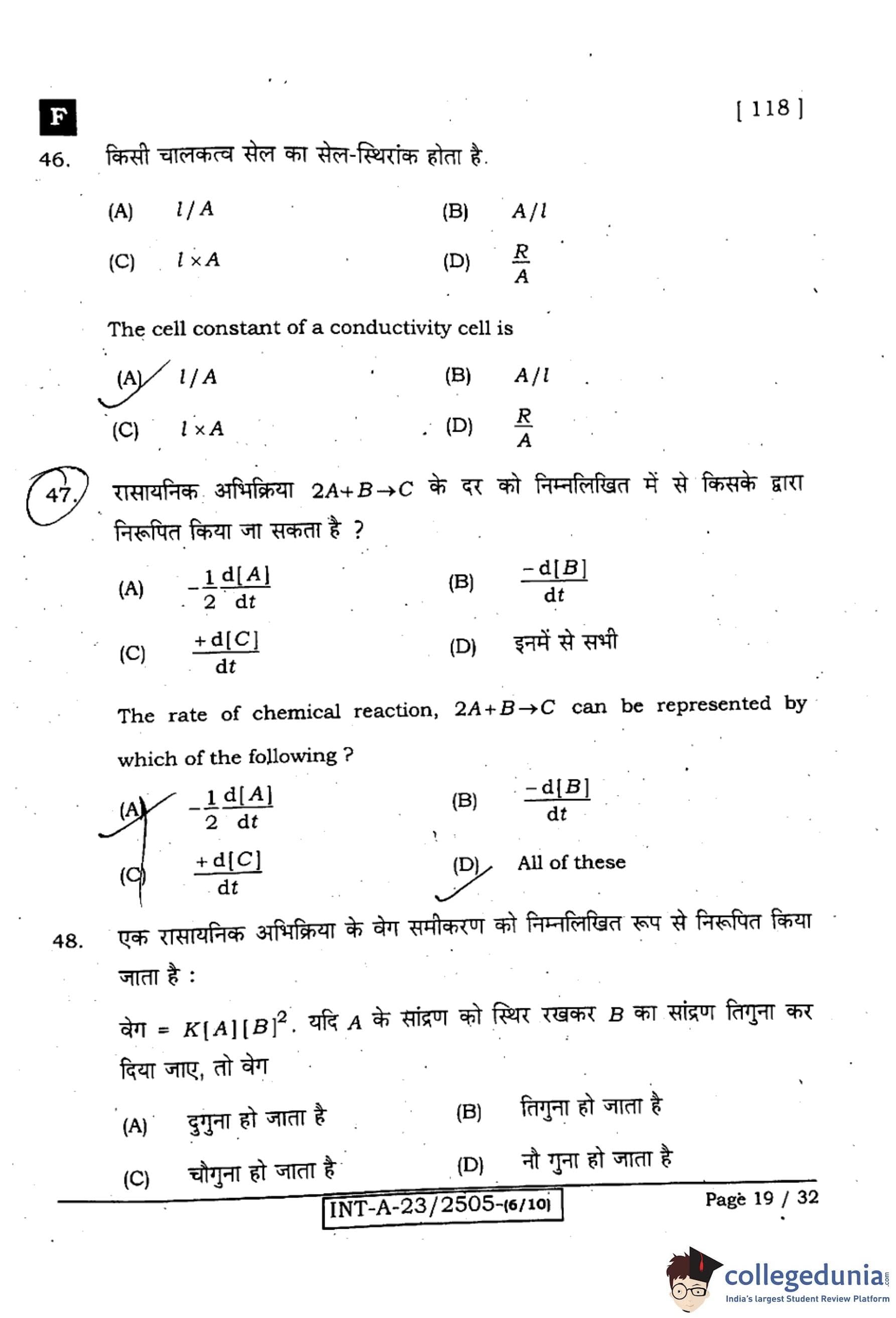













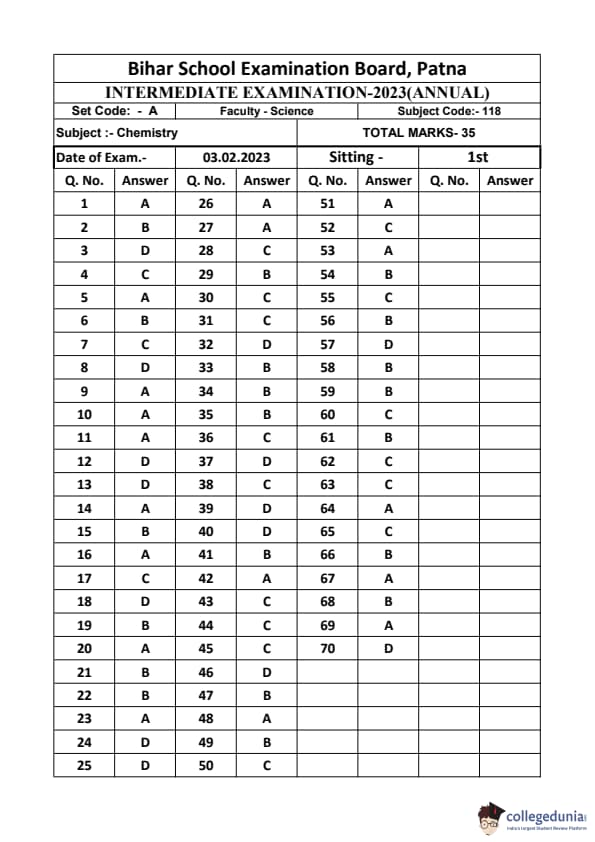
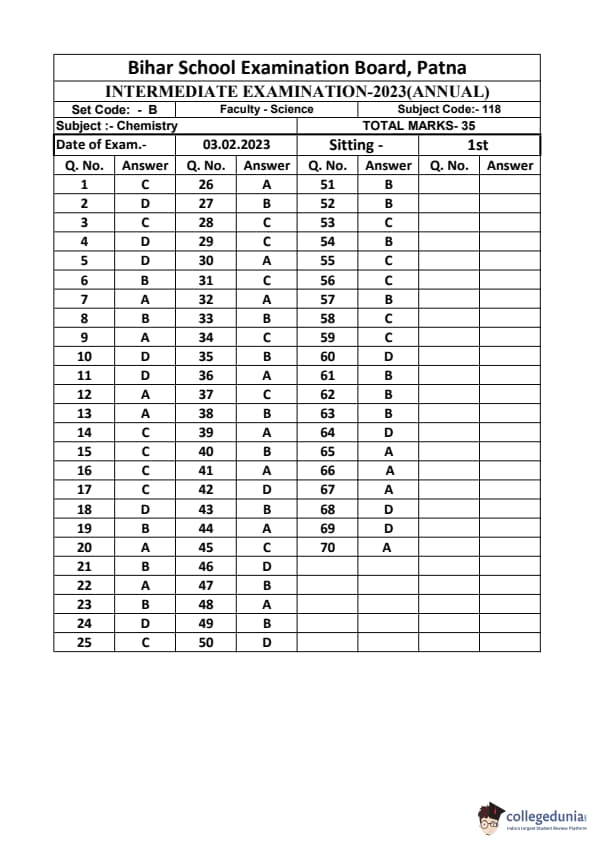
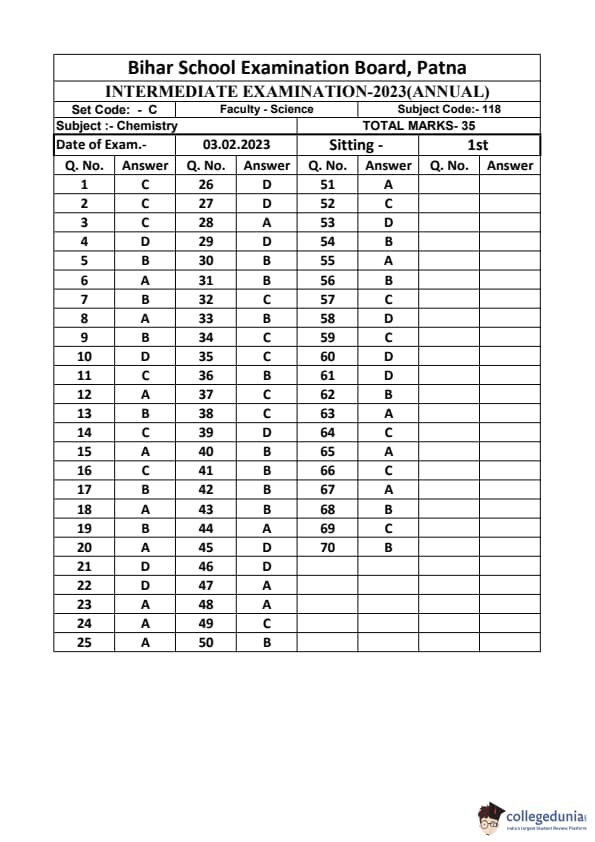
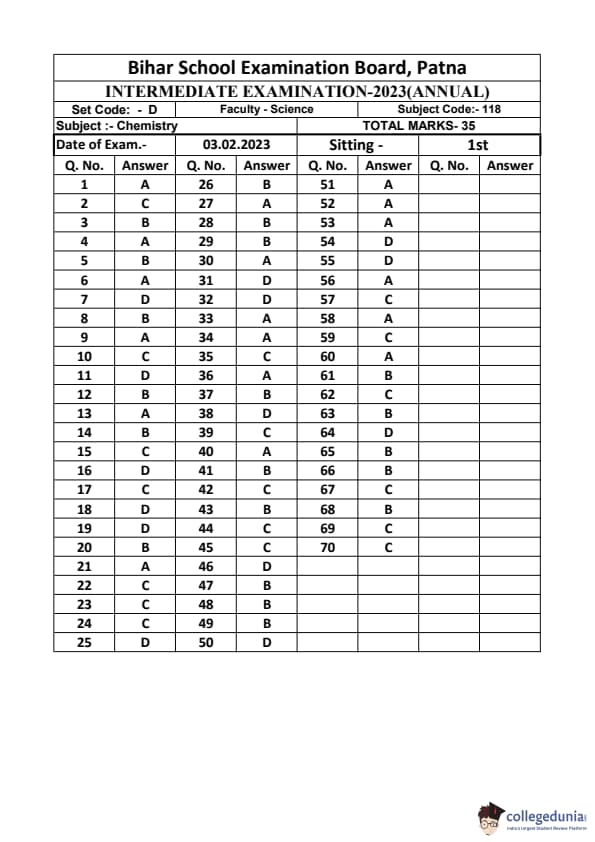
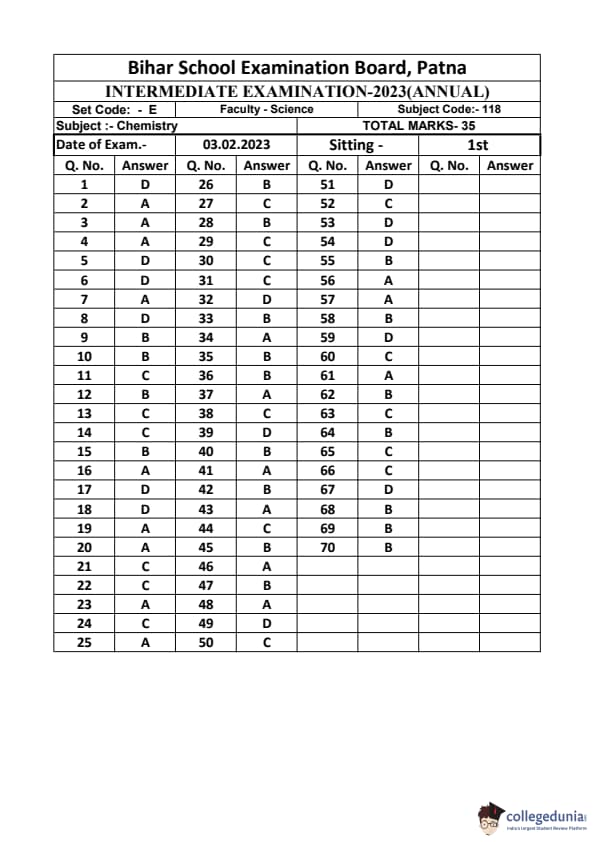
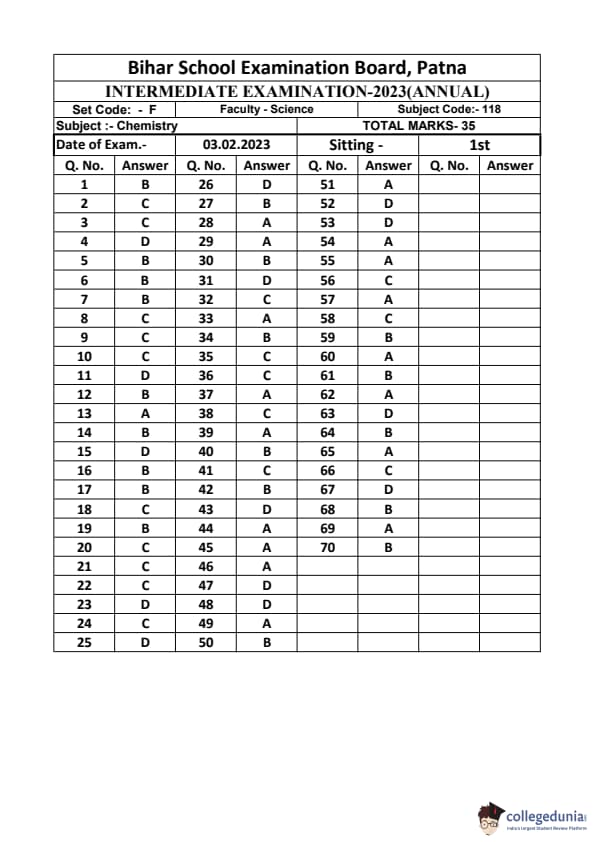
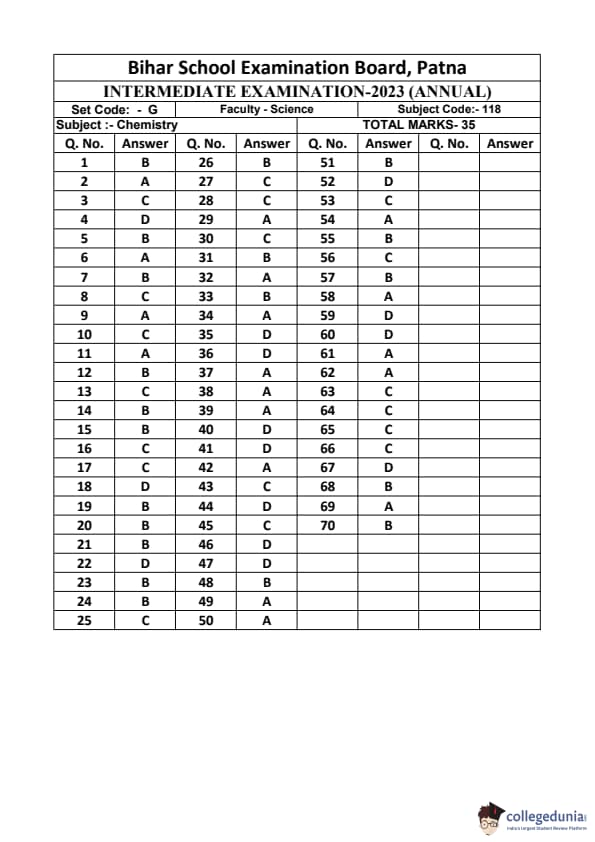
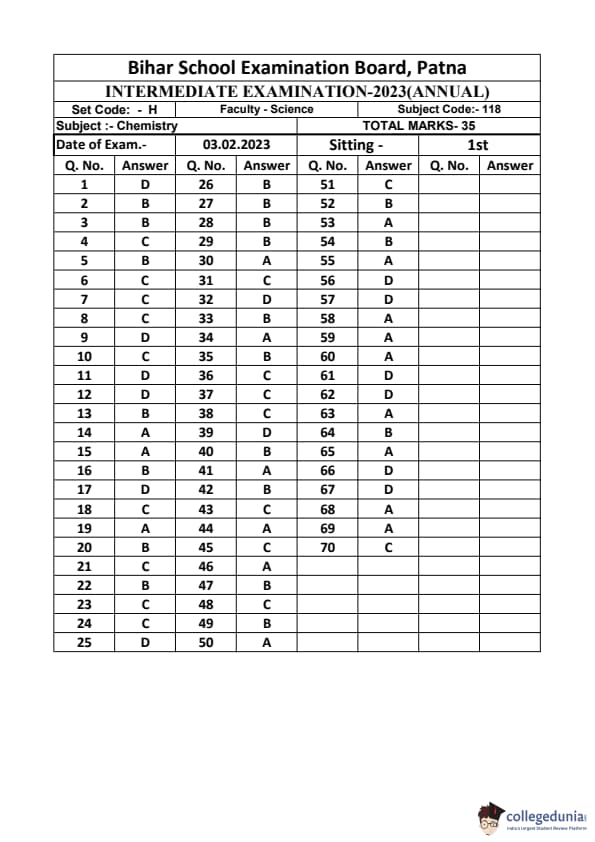
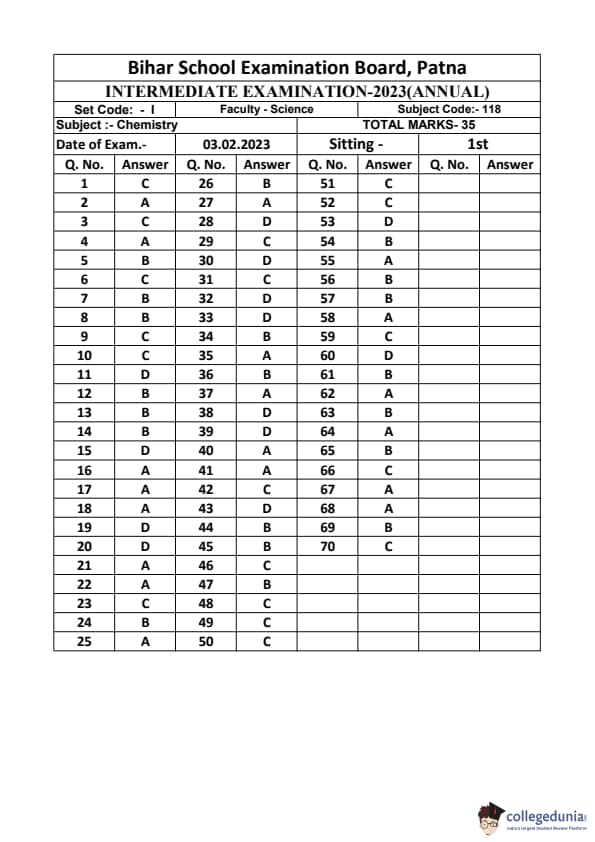
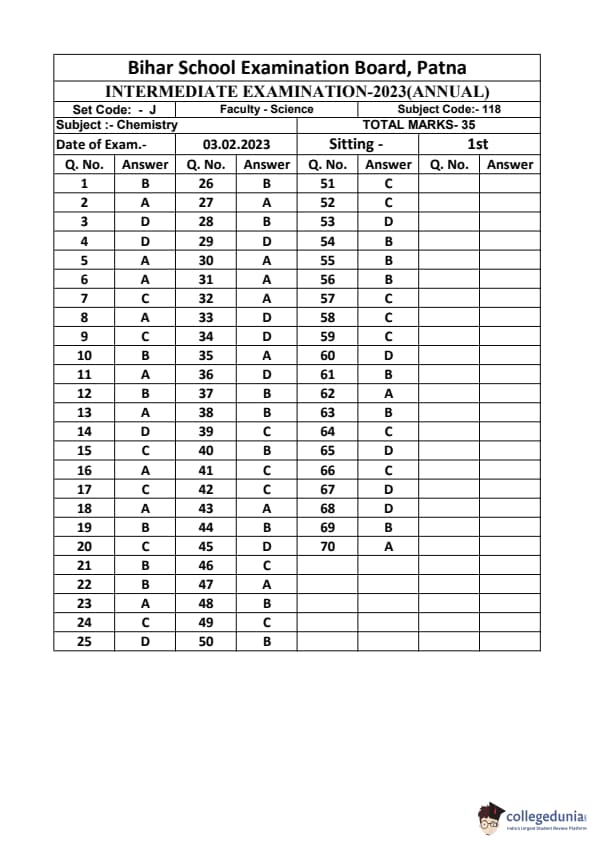





Comments