Jasmine Grover Study Abroad Expert
Study Abroad Expert
Density is the measurement of the mass of an object per unit volume. It is represented by the letter D and the symbol ρ, ‘rho’. The SI unit of density is kg/m³ – unit kilogram per cubic meter. Density can be calculated by its formula which is mass divided by volume. For example, a homogeneous material like ice has the same density throughout as its molecules are closely packed together.
Read More: Surface Tension
What is Density?
The density of a material is defined as the denseness of that particular material in a specific given area.
- The density of the material refers to its mass per unit volume.
- It is generally a measurement of how tightly the matter particles are packed together.
- Various materials have different densities based on the intermolecular forces involved in packing.
- The density of water, i.e. 1 gram/cubic centimeter, is commonly used as the standard value for calculating the density of different substances.

SI Unit of Density
Density is measured with the SI unit as Kilograms per cubic meter (kg/m3). Density definition – Density is defined as the relationship between the substance’s mass and the volume it occupies.
In qualitative terms, it indicates how heavy an object can be at constant volume. The density of different substances is different which means that the different substances with similar volumes will weigh differently.
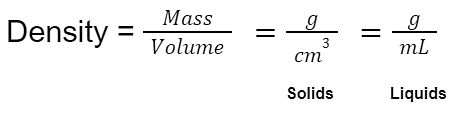
Unit of Density for Liquids and Solids
Also Read: Properties of Fluids
Other Units of Density
If we talk about other density units, metric tons and liter are also used despite the fact that they are not part of the SI unit system. The other units of density are as follows:
- Gram per millilitre (g/mL)
- Gram per cubic centimeter (g/cm3) -------- 1 g/cm3 = 1000 kg/m3
- Kilogram per cubic decimetre (kg/dm3)
- Metric ton per cubic meter (t/m3)
- Kilogram per litre (kg/L)
- Megagram (metric ton) per cubic meter (mg/m3)
Even though the SI unit of density is kg/m³, the other commonly used units to measure density are:
- Solids - g/cm³
- Liquid - g/ml
- Gases - g/L

Furthermore, the CGS unit of density is gram per cubic centimeter (g/cm3).
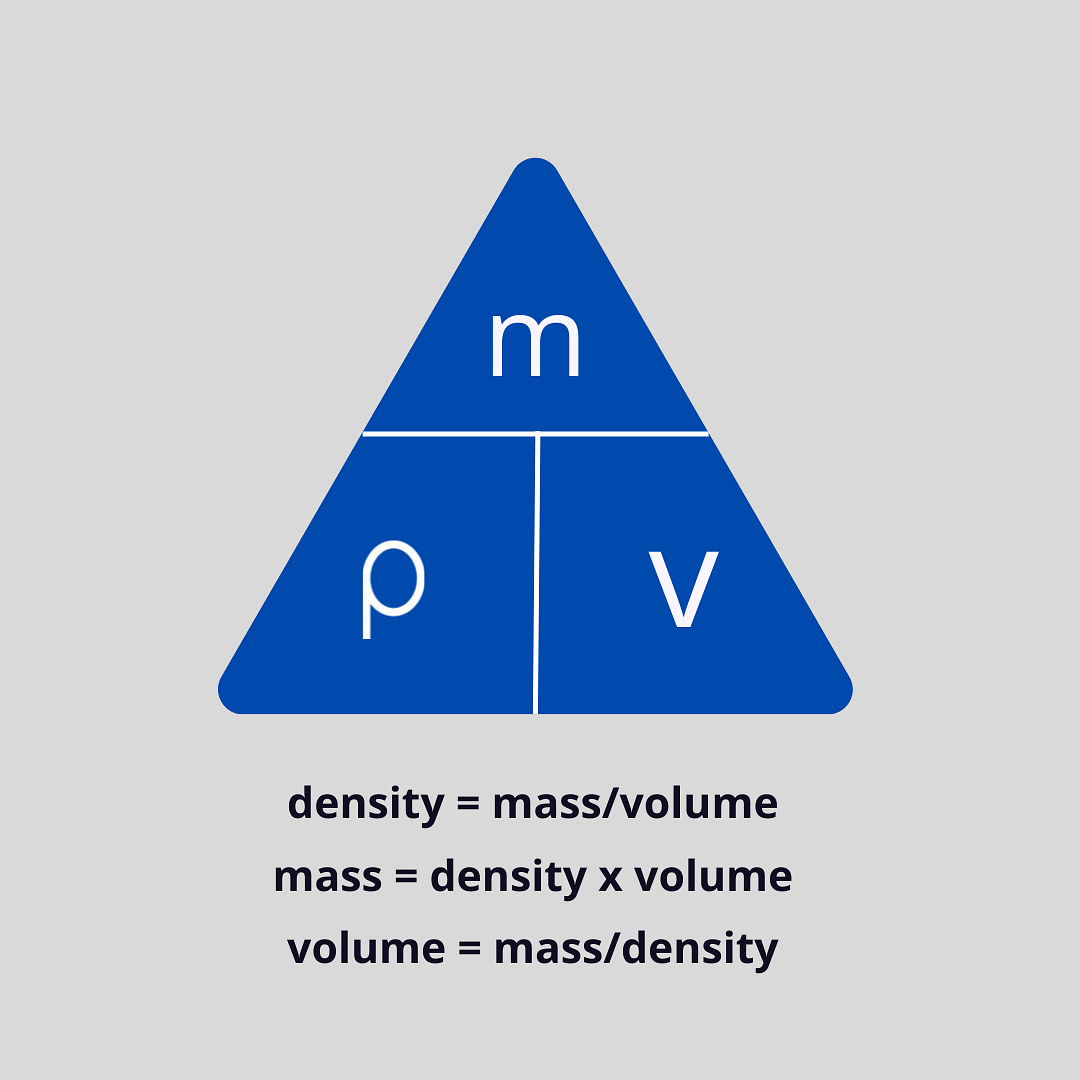
Density Formula
The density of an object can be calculated by the formula:
ρ = m/v
Where Density symbol represents,
- ρ = Density of the object
- m = Mass of the object
- v = Volume of the object
The particles in solids are tightly packed with very little space between them, therefore the solids are denser than liquids and gases. The liquids are less dense than solids because the particles are not tightly packed in liquid but denser than gases which contain free-flowing particles.
Density Formula
Solved ExampleExample 1: Calculate the density of water if its mass is 1160 Kg with a 1 m3 volume. Solution: Given, Mass = 1160 Kg Volume = 1m3 Density = Mass/Volume ρ = 1160/1 = 1160 kg/m3 Example 2: If a shiny rock, a carbon allotrope with a 0.042 cm3 volume and 0.14 g mass is given, is it graphite or diamond? The density of diamond is 3.51g/cm3 and the density of graphite is 2.266 g/cm3. Solution: Given, Volume of the rock = 0.042 cm³ Mass = 0.14 g Density of diamond = 3.51g/cm3 Density of graphite = 2.266 g/cm3 Using the density equation, we get: ρ = m/V m = ρV m = 2.266 g/cm³ x 0.042 cm³ = 0.0951g for graphite m = 3.51g/cm3 x 0.042 cm³ = 0.1474 g for diamond The mass of the shiny rock is identical to the mass of a diamond. |
Examples of Density
To understand the concept of density better, let us look at a couple of examples:
- Lead, iron, and platinum are various examples of dense materials. Materials with high density are harder and they are more “likely” to feel heavy.
- Aluminium, glass, and bamboo are some examples of sparse materials which are the opposite of dense materials.
Let us look at the densities of some substances:
| Substance | Density |
|---|---|
| Naphtha | 0.7 g/cm3 |
| Ice | 0.92 g/cm3 |
| Mercury | 13.6 g/cm3 |
| Foam Rubber | 28 kg/m3 |
| Helium Gas | 0.000178 g/cm3 |
| Uranium | 18.7 g/cm3 |
Generally, we can observe that solids are denser than liquids and liquids are denser than gases. The reason behind this is that the particles in solids are densely packed. For liquids, the particles are somewhat loose and can slide across each other while for gases the particles are loose and free to move around.
Types of Density
The density is classified into two types as follows:
- Relative Density: The relative density is also known as specific gravity. It is defined as the ratio of the material’s density to the reference material’s density. It does not have units.
- Absolute Density: The mass of any substance per unit volume of a material is defined as absolute density. The unit of absolute density is g/cm3.
Also Read: Specific Gravity Formula
Applications of Density
The real-life applications of density include:
- Consider two balloons, one is filled with air while the other is filled with coke. Coke is much denser because its atoms move around less and take up less space. On the other hand, in an air balloon, the atoms collide with each other very quickly and therefore take up more space or less density. As a result, the balloon filled with air is light and the balloon filled with coke is heavy.
- Life Tubes: The life tubes are found in swimming pools or beaches and are designed in such a way that they float in water and protect people from drowning while swimming. Life tubes float because they are filled with air which is less dense than water.
- Ships: Ships can float in water because they are designed with ballast tanks for holding air. These tanks offer large volumes with little mass which thereby decreases the ship’s density. When this reduced density is combined with the buoyant force it allows the ship to float over water.
Also Check:
Things to Remember
- Density is defined as the measurement of the mass of an object or body with a unit volume.
- The density of an object can be calculated by the formula: ρ = m/v
- Solids are denser than liquids and liquids are denser than gases.
- The density of water, i.e. 1 gram/cubic centimeter, is commonly used as the standard value for calculating the density of different substances.
- The CGS unit of density is gram per cubic centimeter (g/cm3).
- The density is classified into two types namely, absolute density and relative density.
- Life tubes and ships are two applications of the concept of density.
Previous Year Questions
- A solid sphere falls with a terminal velocity of 20m/s in air. If it is allowed to fall in a vacuum…? [AMUEEE 2015]
- If the excess pressure inside a soap bubble is balanced by an oil column of height…? [JKCET 2004]
- A uniform capillary tube of inner radius r is dipped vertically into a beaker filled with water…? [JEE 2018]
- Consider a soap film on a rectangular frame of wire of area 4×4cm2. If the area of the soap…? [AMUEEE 2016]
- If the work done in blowing a bubble of volume V is W, then the work done in…? [BCECE 2003]
- Bernoulli’s principle is based on the law of conservation of…? [UPSEEE 2010]
- If two soap bubbles of different radii are connected by a tube…? [BCECE 2004]
- An open U-tube contains mercury. When 11.2cm of water is poured into one of the arms…? [BCECE 2006]
- At what speed, the velocity head of water is equal to the pressure head of 40cm of Hg…? [BCECE 2008]
- A sphere of radius R is gently dropped into the liquid of viscosity η in a vertical uniform tube…? [JKCET 2008]
- A water drop is divided into 8 equal droplets. The pressure difference between… [BHU UET 2008]
- A tank is filled with water. There is a hole in the bottom. At the bottom total pressure…? [BCECE 2014]
- Which one of the following equations is Torricelli law? [JKCET 2014]
- Work done in increasing the size of a soap bubble from a radius of 3cm to 5cm is nearly…? [AMUEEE 2016]
- If the potential energy of a body on a planet is numerically U and the escape velocity…? [BHU UET 2008]
- A square wire frame of size L is dipped in a liquid. On taking out, a membrane is formed…?[DUET 2004]
- Two capillaries of lengths L and 2L and of radii R and 2R are connected in series…? [JCECE 2010]
- A closed compartment containing gas is moving with some acceleration in a horizontal direction…? [JCECE 2010]
- A rain drop of radius 0.3mm has a terminal velocity of 1m/s and the viscosity of…? [BCECE 2003]
- A cylindrical capillary tube of 0.2mm radius is made by joining two capillaries…? [JEE 2019]
Sample Questions
Ques 1. How will we find out if a substance is less dense than water? (1 mark)
Ans. If the weight of a substance is less than an equal volume of water, it is less dense and will float.
Ques 2. What will be the density of water if its volume is 1m3 and mass is 1160 Kg? (3marks)
Ans. It is given that:
Mass (m) = 1160 Kg
Volume (v) = 1m3
So the density will be:
Density (ρ) = Mass (m)/Volume (v)
= 1160/1
= 1160 kg/m3
Ques 3. Calculate the volume of a rock if its mass is 60g and density is 2 g/cm3. (3 marks)
Ans. Density (ρ) = 2 g/cm3
Mass (m) = 60 g
Density = Mass/Volume
So, Volume = Mass/Density
Substituting these values in the formula,
Volume = 60/2
= 30 cm3
Ques 4. What will be the mass of a diamond if its density is 3.5 g/cm3 and volume is 0.5 cm3? (3 marks)
Ans. Density (ρ) = 3.5 g/cm3
Volume (v) = 0.5 cm3
We know that Density = mass/volume
So, Mass = Density * volume
Substituting these values in the formula,
Mass = 3.5 * 0.5
= 1.8 g
Ques 5. Suppose there are two boxes of the same volume, one box is filled with x balls and the other is filled with 6x balls. If each ball is of the same mass, which box will weigh more? (3 marks)
Ans. The box with the greater number of balls will have more mass per unit of volume.
No. of balls in box 1 = x balls
No. of balls in box 2 = 6x balls
Since the balls in the second box are 6 times more than the first box, the second box would weigh more.
Ques 6. How would you find the density of a human body? (3 marks)
Ans. The density of a human body can be calculated by the formula:
Density = Mass/Volume
A weight scale can be used to calculate the mass of the human body. And, the volume can be calculated by using the submersion displacement. To calculate volume, fill the tub with water and allow the person to fully submerge in that. After that, there will be a rise in the level of water, this rise will be the volume of the body. So, you can easily calculate the density by putting the values in the above formula.
Ques 7. Calculate the density of a liquid with 2000 kg mass and a volume of 2 m3. (3 marks)
Ans. Given, Mass – 2000 kg
Volume – 2 m3
The formula for determining density is: Mass/Volume
1160 kg/m3 = 2000/2 = 1000 kg/m3
Ques 8. Calculate the density of rock of 45 gm mass and a volume of 15 cm3. (2 marks)
Ans. As we know, density = mass/volume
Density = 45/15
= 3 gm/cm3
Ques 9. Calculate the density of 30 mL of a solution that weighs 120 grams. (2 marks)
Ans. \(\rho = {120 g \over 30 mL}\)
Density = 4 g/mL
Ques 10. Calculate the density of 0.4 L of solution weighing 150 grams in g/mL. (3 marks)
Ans. The density is to be determined in g/mL, but our volume is in liters, so firstly we convert the volume to mL.
V = 0.4 L x 1000mL/1L
= 400 mL
Now, density = mass/volume
= 150 g/400mL
= 0.375 g/mL
Ques 11. What is SI unit of density? (1 mark)
Ans. The SI unit of Density is measured with kilograms per cubic metre (kg/m3).
Ques 12. The density of brass is 8.4 g cm−3. What do you mean by this statement? (1 mark)
Ans. This statement means that one cubic centimetre volume of brass has a mass of 8.4g.
Do Check Out:





Comments