Collegedunia Team Content Curator
Content Curator
Unit of pressure is commonly expressed in terms of Pascals. Pressure is defined as the perpendicular force force applied per unit area. Pressure is a scalar quantity and is exerted by all three states of matter, solids, liquids, and gases. When pressure is measured relative to the ambient pressure, it is called as Gauge pressure.
Based on the applications, pressure is measured using different system of units. N/m2 or Pascal is the most commonly used system of unit of pressure. Pound-force, psi, atm, torr, etc. are some of the other used unit of pressure. Based on the height of a specific fluid in the manometric column, unit of pressure is also expressed as cm of water, mm of Hg, etc.
Also Read: International System of Units
Key Terms: Pressure, SI Unit, CGS Unit, Barye, Atmosphere, Bar, Pascal, Torr, Perpendicular Force, Area
Pressure
[Click Here for Sample Questions]
Pressure is perpendicular force acting per unit area. It is denoted by the letter ‘P’. Pressure is expressed by the formula
P = F/A
Where, P- Pressure Applied
F- Perpendicular force acting
A- Area of cross-section
Pressure acts as a fundamental driving force for many natural and man-made phenomena. Pressure is the reason for the formation of cyclones and hurricanes, it also acts as the core principle for many modern inventions such as hydraulic power jacks and rocket propulsion systems.

Pressure is also referred to as Stress. Unit of Pressure is most commonly expressed in SI units as N/m2, or Pascals (Pa). The dimensional formula is given by ML-1T-2. Atmospheric pressure is measured to be close to 100,000 Pa. Different systems have been used to express uinit of pressure based on the applications.
SI Unit of Pressure
[Click Here for Sample Questions]
The SI system of units is a modern version of the metric system. It is a widely accepted standard that was adopted from French Système international (d’unités). It consists of seven fundamental quantities from which the derived quantities are observed.

The SI unit of pressure is Pascal (Pa) named after the scientist Blaise Pascal. This unit is commonly used to express the force exerted on materials such as stress, strength, elastic modulus, and hardness. Therefore people involved in the field of material science are used to this system of the unit. One Pascal pressure is equal to one Newton per square meter (N/m2 or kgm-1s-2).

By using appropriate prefixes adopted from the metric system, larger pressure values can be represented in terms of MPa (Mega pascals) GPa (Giga pascals).
Also Read: Mechanical Properties of Fluids
CGS Unit of Pressure
[Click Here for Sample Questions]
Barye (Ba) is the CGS unit of pressure. It is not standardized and is called by many names worldwide such as baryd, baryed, barad, barrie, barie, or bary.
It is expressed as 1 dyne per square centimeter (dyn/cm²). Dyne refers to the force required to accelerate the mass of 1g to the rate of 1 cm per second.
Alternatively,
1 Pa = 0.1 Ba
Also Check:
Other Units of Pressure
[Click Here for Sample Questions]
Pounds per square inch, bar, atm, cm of water, mm Hg, etc. are some of the other most commonly used units of pressure. The following figure shows the conversion units among the different SI Units of Pressure used.

Standard Atmospheres (atm)
- Standard atmosphere (atm) is the unit of pressure used to measure the atmospheric pressure.
- Even though the unit of atmospheric pressure is pascal (Pa), standard atmosphere (atm) is used for convenience and better understanding.
- For example, It is more sensible to say that a container has 100 atm (100 times air pressure than the current atmospheric pressure) than saying that the container has 100 pascals of pressure.
- Standard atmosphere can be expressed in terms of pascals as: 1 atm = 101,325 Pa
- This unit of pressure in atm was originally measured for air pressure at sea level at 0°C.
- Atmospheres are normally used when an accurate measurement of pressure is not needed.
- It is an instinctive and coarse unit of pressure measurement.
- Bar is a metric unit of pressure that has been in use especially in meteorology.
- It is derived from the Greek word ‘baros’ meaning weight.
- The unit of pressure, mbar can be easily converted into pascals as 1 mbar is equal to 100 pascals.
- Some of the Vacuum equipment that operates under pressure use units expressed in terms of mbar.
- Bars can be expressed in terms of pascals as: 1 bar = 100,000 Pa
Imperial Units
- Countries such as the United States and the United Kingdom use a system of measurement that can vary from the standard SI unit system.
- Here the weight is expressed as pounds (lb) or ounces (oz) and length is measured in terms of feet (ft) or inches (in).
- Thus by substituting the imperial units in the place of standard units, we can express the unit of pressure as: lbf/ft², ozf/in², pounds per square inch (psi), iwg.
- Pressure is commonly expressed in terms of psi units in the US. Small pressure differences in the shaft and pipes are measured in terms of inches of water gauge (iwg).

Torr
- Torr is a unit of pressure named after Evangelista Torricelli who invented the barometer (device to measure air pressure).
- It is mostly used to measure low-pressure values expressed in terms of milli-torr (mTorr).
- 1 Torr is approximately equal to 1mm Hg (mercury rise when unit pressure acts on it).
- It can be expressed as: 1 atm = 760 Torr or 1 Pa = 0.00750062 Torr
Also Read: Torricelli’s Law
Pièze (pz)
- It is an old system of measurement that was in use since the early 18th century especially in France.
- The pièze is defined as 1 sthène per square meter (sn/m²).
- This unit of pressure is expressed in pascals as: 1 pz = 1kPa.
Technical Atmosphere (at or kgf/cm²)
- This unit of pressure is a non-SI systems of unit that is outdated.
- These are the equivalent of the American system of pressure measurement (psi).
- It is measured in kilogram-force per square centimeter, kgf/cm2.
- Technical atmosphere can be expressed as: 1 at = 0.97 atm

Manometric Units
[Click Here for Sample Questions]
- Manometric units is the unit of pressure measured by the use of liquid rise when pressure force is applied to it.
- It is measured in mm.
- In earlier times, Mercury (Hg) was used as the liquid medium.
- As the temperature and density of liquid vary from place to place, manometer units are not very accurate.
- These include centimeters of mercury (cmHg), inches of mercury (inHg), millimeters of water (mmH2O), centimeters of water (cmH2O), and inches of water (inH2O or iwc).
- Deep-sea divers use meters of seawater (msw) or feet of seawater (fsw).
- The unit of pressure are calculated by taking average values of the density of seawater and gravity.

Also Read: Value of R in atm
Unit of Pressure Conversion- ExamplesExample 1: Convert 0.357 atm to torr Solution: Using the relation 760 torr = 1atm \(= 0.357 atm \times \frac{760 torr}{1 atm} \) = 270 torr Therefore, 0.357 atm = 270 torr Example 2: Convert 147.2 kPa to torr Solution: Using the relation 760 torr = 101.325 kPa \(= 147.2 kPa\times\frac{760 tor}{ kPa}\) = 1104 torr Therefore, 147.2 kPa = 1104 torr |
Handwritten Notes on Pressure
[Click Here for Sample Questions]
Provided below are some quick handwritten notes on basic principles of Pressure, Pascal’s Law, Hydraulic Lift, Atmospheric Pressure, Torricelli’s Experiment and Archimedes Principle.
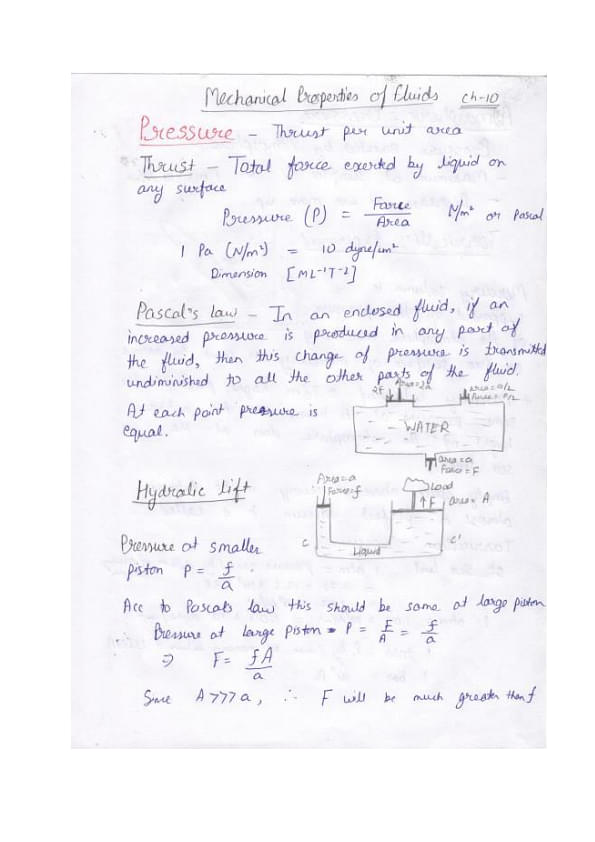



Things To Remember
[Click Here for Sample Questions]
- The SI unit of pressure is pascal (Pa), N/m2.
- Pressure is the perpendicular force acting on the cross-sectional area.
- The CGS unit of pressure is barye (Ba).
- Other commonly used units to express pressure measurement are: Torr, at, pz, bar etc.
- Manometric units are those which are measured by the use of liquid rise when pressure is applied to it.
- For deep-sea diving, meters of seawater (msw) or feet of seawater (fsw) is used.
- Pressure is commonly expressed in terms of psi in the US.
Do Check Out:
Previous Year Questions
- Water is supplied to different localities from a tank. What is the water pressure? [JIPMER 1996]
- A solid sphere falls with a terminal velocity of 20m/s in air. If it is allowed to fall in vacuum…? [AMUEEE 2015]
- Bernoulli’s principle is based on the law of conservation of…? [UPSEEE 2010]
- If the excess pressure inside a soap bubble is balanced by oil column of height…? [JKCET 2004]
- An open U-tube contains mercury. When 11.2cm of water is poured into one of the arms…? [BCECE 2006]
- A sphere of radius R is gently dropped into liquid of viscosity η in a vertical uniform tube…? [JKCET 2008]
- If the work done in blowing a bubble of volume V is W, then the work done in…? [BCECE 2003]
- A water drop is divided into 8 equal droplets. The pressure difference between… [BHU UET 2008]
- A cylindrical capillary tube of 0.2mm radius is made by joining two capillaries…? [JEE 2019]
- A square wire frame of size L is dipped in a liquid. On taking out, a membrane is formed…?[DUET 2004]
- A uniform capillary tube of inner radius r is dipped vertically into a beaker filled with water…? [JEE 2018]
- Two capillary of lengths L and 2L and of radii R and 2R are connected in series…? [JCECE 2010]
- Consider a soap film on a rectangular frame of wire of area 4×4cm2. If the area of the soap…? [AMUEEE 2016]
- A tank is filled with water. There is a hole in the bottom. At the bottom total pressure…? [BCECE 2014]
- Which one of the following equation is Torricelli law? [JKCET 2014]
- At what speed, the velocity head of water is equal to pressure head of 40cm of Hg…? [BCECE 2008]
- A rain drop of radius 0.3mm has a terminal velocity of 1m/s and the viscosity of…? [BCECE 2003]
- If the potential energy of a body on a planet is numerically U and the escape velocity…? [BHU UET 2008]
- A closed compartment containing gas is moving with some acceleration in horizontal direction…? [JCECE 2010]
- Work done in increasing the size of a soap bubble from a radius of 3cm to 5cm is nearly…? [AMUEEE 2016]
- If two soap bubbles of different radii are connected by a tube…? [BCECE 2004]
- To what depth does the ball with a certain density and falling from a height sink? [BITSAT 2013]
- What is the pressure exerted by 6 g of methane gas in a 0.03 m3 vessel? [NEET 2010]
Sample Questions
Ques. Which state of matter has the maximum value of the temperature coefficient of cubical expansion? (1 Mark)
Ans. The gaseous phase of matter has the highest value of temperature coefficient of cubical expansion.
Ques. Given a barometric pressure of 27.5 in. Hg, express the pressure in each of the following unit of pressure: (5 marks)
(a) atm
(b) mm Hg
(c) psi
(d) kPa
Ans. a. To express the pressure in atmospheres, we derive a unit factor related to the equivalent relationship 29.9 in. Hg = 1 atm.

- To convert to millimeters of mercury, we derive a unit factor related to the equivalent relationship 29.9 in. Hg = 760 mm Hg

- To calculate the pressure in pounds per square inch, we derive a unit factor related to the equivalent relationship 29.9 in. Hg = 14.7 psi.

- To find the pressure in kilopascals, we derive a unit factor related to the equivalent relationship 29.9 in. Hg = 101 kPa.

Ques. An atmospheric sample contains nitrogen, oxygen, argon, and traces of other gases. If the partial pressure of nitrogen is 587 mm Hg, oxygen is 158 mm Hg, and argon is 7 mm Hg, what is the observed pressure as read on the barometer? (3 marks)
Ans. The sum of the individual partial pressures equals the total atmospheric pressure; therefore,
Pnitrogen + Poxygen + Pargon = Ptotal
Substituting the values for the partial gas pressures, we have
587 mm Hg + 158 mm Hg + 7 mm Hg = 752 mm Hg
Thus, the atmospheric pressure as read on the barometer is 752 mm Hg.
Ques. A steel container filled with nitrous oxide at 15.0 atm is cooled from 2 °C to –40 °C. Calculate the final pressure assuming the volume remains constant. (3 marks)
Ans. We can solve this problem using the equation
Substituting for each variable and simplifying, we have 
Ques. A nitrogen gas sample occupies 50.5 mL at –80 °C and 1250 torr. What is the volume at STP? (3 marks)
Ans. We can solve this problem using the equation
Substituting for each variable and simplifying, we obtain 
Ques. A 275 L helium balloon is heated from 20 °C to 40 °C. Calculate the final volume assuming the pressure remains constant. (3 marks)
Ans. We can solve this problem using the equation


Substituting for each variable and simplifying, we obtain

Ques. A 1.50 L sample of methane gas exerts a pressure of 1650 mm Hg. Calculate the final pressure if the volume changes to 7.00 L. Assume the temperature remains constant. (3 marks)
Ans. We can solve this problem using the equation

Substituting for each variable and simplifying, we obtain

Ques. The air pressure outside a cruising jetliner is approximately 2.3 x 104 Pa. What is this pressure in atmospheres? (3 marks)
Ans. 1 atm = 1.01325 x 105 Pa
Set up the conversion so the desired unit will be canceled out. In this case, we want Pa to be the remaining unit.
pressure in atm = (pressure in Pa) x (1 atm/1.01325 x 105 Pa)
pressure in atm = (2.3 x 104/1.01325 x 105) Pa
pressure in atm = 0.203 atm
Do Check Out:





Comments