
Exams Prep Master
Sodium Chlorate [NaClO3] is an inorganic compound readily soluble in water, it looks like a pale to yellow to white crystalline (sugar-like) powder. This compound is basically known as hygroscopic and its sub-lethal exposure to human beings can cause symptoms like nausea, vomiting, diarrhoea, sore throat, dermatitis etc. It is used in making herbicides, dyes, explosives, cosmetics, pharmaceuticals, paper and other chemicals as well. Sodium Chlorate had widespread use in agriculture as a pesticide and weedicide but this has been banned by European Union as it is injurious to Human as well as environmental health. Moreover, above 300°C, it decomposes to release oxygen and leaves sodium chloride. Here, we will discuss this chemical compound in depth along with some important questions.
| Table of Contents |
Keyterms: Sodium, Chlorate, inorganic compound, nausea, vomiting, diarrhoea, sore throat, dermatitis, oxygen
Also check: Periodic Properties of Elements
What is Sodium Chlorate?
[Click Here for Sample Questions]
Sodium Chlorate is an inorganic compound with a chemical formula of NaClO3 and the compound is hygroscopic salt i.e., its capacity to attract water towards itself from its surroundings via adsorption or absorption is exceptionally high. So, its ability to consistently attract water from its surroundings.

Sodium Chlorate
On chemical grounds, Sodium Chlorate is classified as an oxidising agent, an irritant [for humans] and an environmental hazard. Its appreciably soluble in water and appears as a white crystalline powder.
Structure of Sodium Chlorate
[Click Here for Sample Questions]
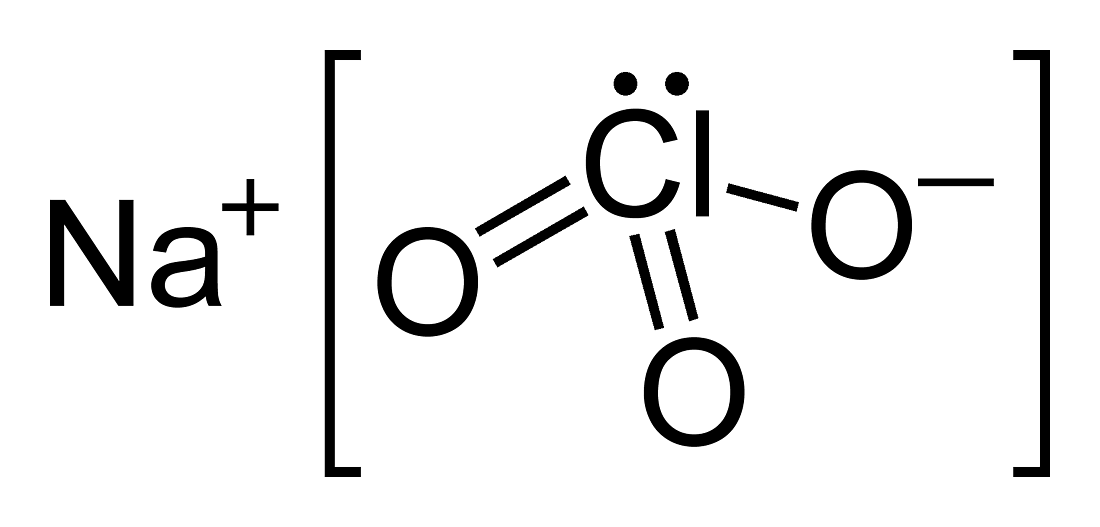
Structure of Sodium Chlorate
Preparation of Sodium Chlorate
[Click Here for Sample Questions]
In order to produce Sodium chlorate, Simple Saltwater is electrolyzed and the reaction is known as the Forrester reaction, it goes something like this:
NaCl + 3H2O + 6e-
NaClO3 + 3H2
This is an exothermic reaction subject to pressure and temperature alterations. It involves multiple steps:
- Chlorine is stored at the anode while hydrogen is stored at the cathode.
- Chlorine is then hydrolysed to a hypochlorite anion group which at one point releases Sodium Chlorate.
- This released Sodium Chlorate are formed in the shape of crystals.
- Then cell liquor [remaining solution obtained after hydrolysis] is separated from the crystal-like depositions and that is how the Sodium Chlorate is obtained.
Also Read:
| Related Articles | ||
|---|---|---|
| Carboxylic Acids | Butanoic Acid | |
| Forms of Water | Francium | |
Physical Properties of Sodium Chlorate
[Click Here for Sample Questions]
Physical properties of sodium chlorate are:
- It’s an odourless solid.
- Its apparency ranges from pale yellow to white crystalline solid
- Its molecular weight is 106.44 g/mol
- Its soluble in water and glycerol and slightly soluble in acetone as well.
- Its heat capacity is 298.15 J/K
- It has a density of 2.49 g/cm3
- Its boiling point is 300° C and melting point is 248° C
- It has a cubic crystal structure.
Chemical Properties of Sodium Chlorate
[Click Here for Sample Questions]
Chemical properties of sodium chlorate are:
- Sodium Chlorate is a powerful oxidising agent. So, its hypochlorite ion helps to oxidize and blech any chemical.
- In reaction with potassium bromide and hydrochloric acid, it gives compounds like potassium chloride, sodium chloride, Bromine and Water
NaClO3 + 6KBr + 6HCl → 6KCl + NaCl + 3H2O + 3Br2
- In reaction with potassium iodide and hydrochloric acid, it gives out sodium chloride, potassium chloride, Iodine and water.
NaClO3 + 6KI + 6HCl → NaCl + 3I2 + 3H2O + 6KCl
- Due to its explosive nature, it is kept in a controlled environment as Sodium chloride will produce flames if brought into contact with wood, sulfuric acid, different metals etc.
Also Read:
| Related Articles | ||
|---|---|---|
| Ferrous Sulphate | Benzene | |
| Interhalogen compounds | Dehydration of Alcohols | |
| Group 18 elements | Coordination Compounds | |
Applications of Sodium Chlorate
[Click Here for Sample Questions]
Sodium Chlorate has the following uses:
- It is used in bleaching different chemicals
- It’s used in making explosives
- It’s used in the agricultural sector for making fertilizers, pesticides, and weedicides.
- It’s used in the production of different colours, matches, inks, beautifiers, defoliants and calfskin.
- It’s used in Solvay Process along with sulphuric acid.
- It’s used in the large-scale manufacture of dyes as an oxidizing specialist.
- It has uses in pharmaceuticals for the preparation of different kinds of drugs.
- Its most important commercial usage is as bleaching pulp in the production of paper.
Note: although uses of Sodium Chlorate are multiple its chronic exposure can cause harm to human beings and this compound is further classified as an environmental hazard as the compound itself is not combustible but can produce flames if brought into contact with other substances, its explosion releases toxic gases including chlorides and sodium oxides.
Things to Remember
- Sodium Chlorate is an inorganic compound with the standard chemical of NaClO3 and its appearance ranges from pale yellow to white crystalline solid.
- Sodium Chlorate is obtained by electrolysis of simple saltwater.
- Sodium chlorate is a powerful oxidising agent readily soluble in water and causes combustion if comes into contact with other substances especially water.
- Sodium Chlorate is mainly used as an oxidising and/or bleaching agent in paper and dye manufacture, its is also used in the manufacture of fertilizers and pesticides.
- Chronic/sub-lethal exposure to Sodium Chlorate can cause health risks in the human body whose symptoms are redness of the skin, blueness of lips and fingernails, vomiting, diarrhoea etc.
Sample Questions
Ques: What is Sodium Chlorate? (2 marks)
Ans: Sodium chlorate is an inorganic compound that looks like a white crystalline powder. Its standard chemical formula is NaClO3 and it is a powerful oxidising agent. The compound is hygroscopic salt i.e., its capacity to attract water towards itself from its surroundings via adsorption or absorption is exceptionally high. So, its ability to consistently attract water from its surroundings.
Ques: What is the preparation methodology of Sodium Chlorate? (3 marks)
Ans: Sodium Chlorate is produced electrolytically from simple saltwater [sodium chloride]. In this process, the temperature is kept at 70° C and the pH of the process has to be controlled throughout. This is an exothermic reaction subject to pressure and temperature alterations. It involves multiple steps:
- Chlorine is stored at the anode while hydrogen is stored at the cathode.
- Chlorine is then hydrolysed to a hypochlorite anion group which at one point releases Sodium Chlorate.
- This released Sodium Chlorate are formed in the shape of crystals.
- Then cell liquor [remaining solution obtained after hydrolysis] is separated from the crystal-like depositions and that is how the Sodium Chlorate is obtained.
Ques: What is the chemical importance of Sodium Chlorate? (2 marks)
Ans: Sodium Chlorate is a powerful oxidising agent due to its high oxidising potential making it highly useful in the production of explosives and dyes, and a good bleaching agent used in the manufacture of paper.
Ques: What are the major uses of Sodium Chlorate? (3 marks)
Ans: Some of the most common uses of Sodium Chlorate are:
- As a bleaching pulp in the paper industry
- As an oxidising agent in large scale dye manufacture
- In the production of fertilizers and pesticides.
Ques: What are the hazards associated with Sodium Chlorate? (4 marks)
Ans: Sodium chlorate poses two types of risks:
Explosion risk: although Sodium Chlorate can not combust on its own but can induce explosion if brought into contact with other materials and these flames release toxic gasses.
Health risk: Sodium chlorate can cause harm to humans if they are exposed it and its symptoms are: redness of skin and eyes [due to contact], headache, cough, sore throat, blue skin etc. [due to inhalation], diarrhoea, vomiting etc. [due to ingestion]
Ques: What happens when solid sodium chlorate is heated? Write a balanced chemical equation for the same. (2 marks)
Ans: Sodium chlorate decomposes into sodium chloride (solid) and oxygen (gas) on heating. The chemical equation for this reaction is:
2NaClO3 + heat → 2NaCl + 3O2
Ques: What are the harmful effects that sodium chlorate can have on human body? (2 marks)
Ans: If sodium chlorate is exposed for a long time, it may have harmful effects on the human body. It can cause redness of the eyes and skin, sore throat, and abdominal pain. Additionally, it can also cause blue lips or skin and medical conditions such as diarrhea, nausea, vomiting, shortness of breath, and unconsciousness.
Ques: Is sodium chlorate acid or base? (4 marks)
Ans: Sodium chlorate has some unique properties which come as an exception to the rule. It is an extremely powerful oxidising agent, solid corrosive and has a pKa of around -1 (the pKa of acidic corrosive is more ike 5 and water is 16). As we know that HCLO3 is an extremely corrosive and powerful ion, we can recognize -CLO3 as its subsequent conjugate base as well as can reason that it is likely a powerless conjugate base of it. Inspite of its strong ion present, it is neutralised by the opposing ionic charges and therefore, forms a weak base.
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Check-Out:





Comments