
Content Curator
Law of conservation of energy states that the total energy of an isolated system always remains constant. It can neither increase nor decrease, but can be converted from one form to another. Energy exist in different forms on the earth like mechanical energy, kinetic energy, heat energy, chemical energy etc. Different types of energy is used to carry out various processes. During a process, one form of the energy gets converted to the other form. For example, chemical energy is used to perform a chemical reaction and as a result of the reaction, heat is evolved. In this process, chemical energy is being converted into heat energy.
Read More: Principle of Conservation of Energy
| Table of Content |
Key Terms: Energy, Law of Conservation of Energy, Kinetic Energy, Potential Energy, Work Done, Equations of Motion
What is Law of Conservation of Energy?
[Click Here for Sample Questions]
Law of conservation of energy states that energy can neither be created nor destroyed. The overall energy of an isolated system remains the same when all forms of energy are taken into consideration. Energy remains constant due to the fact that one form of energy can be transformed into another. Law of conservation of energy applies to all the forms of energy present in the universe.

If there is a consumption of energy or loss of energy in one section of an isolated system, such as the universe, there must be an equal amount of energy gained in another. The equation for the amount of energy in any system is as follows;
UT = Ui + W + Q
Where,
- UT refers to the total energy of a system
- Ui refers to the initial energy of a system
- Q is the heat gained or lost by the system
- W is the work done by or on the system
During a work done, internal energy of a system changes. This change in internal energy of a system can be calculated using below equation;
ΔU = W + Q
where, ΔU is the change in internal energy of the system.
Derivation of Law of Conservation of Energy
[Click Here for Sample Questions]
Let us take an example of an object falling on the ground. Assume the potential energy at the earth’s surface to be zero.
Consider a position A that is at a height ‘H’ above the ground, the velocity of the object is zero, hence potential energy is highest at this point.
E = mgH ———- (1)
When the object falls, its potential energy decreases but its kinetic energy increases.
The object falls freely due to gravity and is at a height "X" above the ground at point B. At this point, it will contain both kinetic and potential energy.
E = K.E + P.E
P.E = mgX ——— (2)
According to the third equation of motion,
v2 = 2g (H – X)
½ mv2 = ½ m. 2g (H – X)
K.E = ½ m. 2g (H – X)
K.E = mg (H – X)——– (3)
Using equations (1), (2) and (3)
E = K.E + P.E
E = mg (H – X) + mgX
E = mg (H – X + X)
E = mgH
Similarly, if we take energy at point C, which is at the bottom, we get mgH. As the object falls, the potential energy of the object is being converted into kinetic energy. There must be a point at which kinetic energy becomes equal to the potential energy of the falling object. Let us assume this point to be at a height ‘x’ from the ground. At this point,
K.E = P.E
P.E = K.E = E/2 ——– (4)
The body is at the height X from the ground,
P.E = mgX ——— (5)
Using equation (4) and (5), we get,
mgX = mgH/2
X = H/2
So, H/2 is the new height.
Energy ConservationEnergy conservation refers to the use of energy resources in a sustainable way. The demand for these resources should not exceed its supply in order to avoid the shortage of resources. Also, there should be ways for rebuilding the resources. Though we can convert one form of energy to another but the process costs time and money. These processes have their own challenges and limitations also. Therefore, sustainable use of energy resources is very important. Energy resources can also be conserved by finding alternative solutions for the resources. For example, use of solar panels helps in saving electrical energy as it generates electricity with the help of heat energy of the sun. |
Read Also:
Examples of Law of Conservation of Energy
[Click Here for Sample Questions]
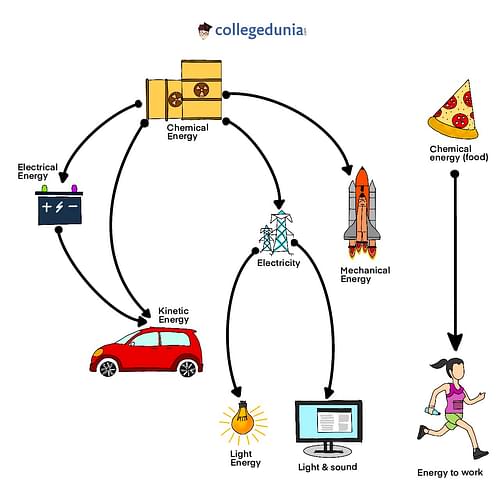
In physics, law of conservation of energy is considered to be a very important law as many processes are based on the fact that energy is always conserved during a process. Numerous electrical and mechanical devices work on the principle of law of conservation of energy. Some common applications of this law can be observed in the following examples:
- In hydroelectric power plants, water falls on the turbine which causes turbines to rotate and generate electricity. Here, the potential energy of water is converted into kinetic energy of the turbine which is ultimately converted into electrical energy.
- Electrical energy gets converted to sound energy in loudspeakers.
- In generators, mechanical energy is used to produce electrical energy.
- During the process of burning of fuels, the chemical gets converted into heat and light energy.
- When food is broken down in the body, chemical energy is transformed to thermal energy, which is utilised to keep the body warm.
- In a torch, chemical energy is transformed into electrical energy, which is then converted to light and heat energy.
Things to Remember
- Law of conservation of energy states that in an isolated system, the total energy remains constant.
- Energy can be converted from one form to the another. For example, in microphones, sound energy is converted to electrical energy.
- All the forms of energy available in the universe follows the law of conservation of energy.
- The total energy of a system can be determined by the equation, UT = Ui + W + Q.
- Whenever a work is done on or by the system, the internal energy of the system changes and is given by, ΔU = W + Q
Sample Questions
Ques. What is the law of energy conservation? (1 Mark)
Ans. In Physics, the law of conservation of energy is an empirical law. It says that in an isolated system, the total amount of energy remains the same with the period of time.
Ques. Where does energy conservation fall short? (1 Mark)
Ans. Energy cannot be created or destroyed as per the law of conservation of energy. It is a universal law that works for all “earthly” observations. The only exceptions to this law can be found in far away galaxies and stars.
Ques. Which energy conversion is taking place when a block slides down a slope? (1 Mark)
Ans. When a block slides down a slope, potential energy is converted into kinetic energy.
Ques. Do gadgets with efficiency less than one break the law of energy conservation? (2 Marks)
Ans. Devices with an efficiency less than one do not break the law of energy conservation. Since some energy converts into less useful energy types, device efficiency is less than one. As a rigid and frictionless system is not there, some energy is lost due to heat or friction but it cannot be destroyed.
Ques. Does gravity conserve energy? (2 Marks)
Ans. Gravity is “unlimited” in the sense that it never stops working. As long as the earth has mass, its gravity will never ever go away. However, as gravity is a force rather than an energy, its never-ending nature cannot be used to extract infinite energy, or any energy at all.
Ques. What is the distinction between law of conservation of matter and law of conservation of energy? (3 Marks)
Ans. The principles of conservation of matter and energy are two laws that are used to explain the behaviour of closed thermodynamic systems that are isolated. These laws define that matter and energy cannot be generated or destroyed, but that they can be changed or rearranged into various forms.
The law of conservation of matter differs from the law of conservation of energy in that the former states that the total mass within a closed system should be constant, whereas law of conservation of energy stipulates that energy cannot be created or destroyed, but can be converted from one form to another.
Ques. State some examples of the law of conservation of energy. (3 Marks)
Ans. Some examples of law of conservation of energy are as follows:
- In a loudspeaker, electrical energy is converted into sound energy.
- Mechanical energy is converted into electrical energy in a generator.
- Chemical energy is transformed into heat and light energy when fuels are burned.
Ques. Can magnets produce gravity? (3 Marks)
Ans. Magnets have two poles, and magnets with opposite poles produce an attractive force. This demonstrates how magnets can be used to generate artificial gravity. However, in order to generate such gravity, the magnets must be sufficiently powerful. The devices used to simulate gravity changes range from centrifuges to “vomit comets,” but simple magnetism is perhaps the most versatile.
Ques. What is the significance of energy conservation? (3 Marks)
Ans. Energy conservation is critical for a variety of reasons.
- It reduces our reliance on non-renewable energy resources such as fossil fuels.
- It also helps save money on energy costs such as utility bills and other energy bills.
- It helps to maintain the continous supply of the resources for the growing demand.
Check Out:





Comments