Collegedunia Team Content Curator
Content Curator
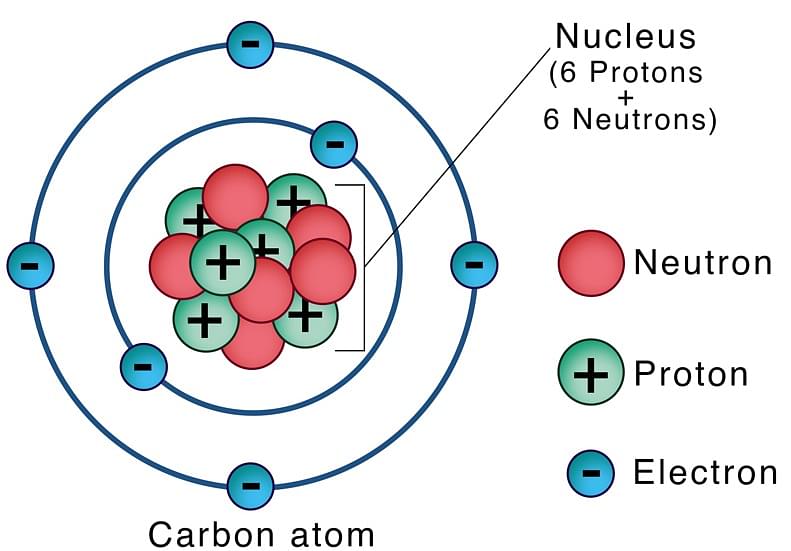
Neutrons are the particles present inside the nucleus of an atom. They are found in all the atoms except hydrogen and are referred to as subatomic particles. Protons and neutrons together constitute the nucleus of an atom. Electrons revolve around the nucleus in fixed orbits and this makes up a general structure of an atom. Thus, an atom is made up of three subatomic particles: Protons, Neutrons and Electrons.
Key Takeaways: Nucleons, Nuclear Force, Protons, Quarks, Alpha Radiations, Deuteron, Gamma Radiations, Beta Decay, Nuclear Reactions
What are Neutrons?
[Click Here for Sample Questions]
Neutrons are neutral subatomic particles found in all atoms except hydrogen. Together with protons, they constitute an atomic nucleus. Protons and neutrons are together called Nucleons. They are bound together by a strong force called the Nuclear Force. Neutrons are essential for the stability of a nucleus.
Atom Structure
Neutrons are made up of units called quarks. It contains two down quarks and one up quark. Neutrons are classified as hadrons (strongly interacting composite particles) and baryons (due to the presence of valence quarks). Quarks are bound together by a strong force resulting due to exchange of particles called gluons.
Neutron is denoted by the symbol ‘n’. The number of protons in an atomic nucleus determines the atomic number (Z) of an atom, whereas atomic mass (A) is calculated by adding the number of protons and number of neutrons present in the nucleus.
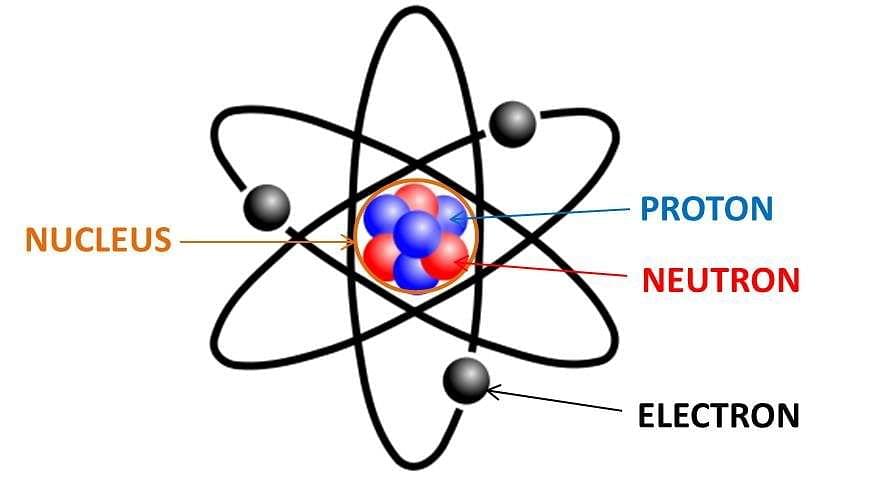
Proton and Neutron
Also Read:
Charge and Mass of a Neutron
[Click Here for Sample Questions]
Charge: Neutrons are electrically neutral. They are neither positively charged nor negatively charged (like protons and electrons respectively). The net charge of a neutron is always 0. This is because a neutron is made up of two down quarks containing a charge of -1/3e and one up quark with a charge of +2/3e.

Charge of Atom
Mass: The mass of a neutron is estimated to be 1.674923 × 10-27 kg. This mass is slightly greater than that of protons. As there is no electric charge on a neutron, its mass cannot be determined by mass spectrometry. So, the mass of a neutron is determined by the help of a deuterium atom. The nucleus of the deuterium is called deuteron. It is made up of a proton and a neutron. The mass of both deuteron and proton can be calculated by mass spectrometry. Thus, the mass of a neutron can be determined by subtracting the mass of a proton from the mass of a deuteron.
Discovery of Neutrons
Neutrons were discovered by British physicist James Chadwick in 1932. But way back In the year 1931, Walther Bothe and Herbert Becker conducted an experiment in which alpha particle radiations from polonium were allowed to fall on beryllium or boron. As a result, an unusual penetrating radiation was generated. Since it was not affected by electric fields, they thought it to be gamma radiation.

Neutron Discovery Experiment
With the studies of these experiments, James Chadwick conducted an experiment in which the penetrating radiation emitted by beryllium sheet was made to fall on paraffin wax. The resulting protons were observed carefully in an ionisation chamber. The interaction of this penetrating radiation with several other atoms was also studied and then it was concluded that this radiation consists of uncharged particles. These uncharged, neutral particles were named neutrons by James Chadwick. Later, he was also awarded the Nobel Prize for this discovery.
Read More: Energy Level Diagram
Properties of Neutrons
[Click Here for Sample Questions]
The general properties of neutrons are as follows:
- Neutrons are electrically neutral particles with zero net charge.
- Neutrons are influenced by magnetic fields but not by electric fields.
- The mass of an electron is negligible as compared to that of a neutron.

Quarks in Proton and Neutron
- Neutrons are essential for the stability of the nucleus.
- Since the mass of a proton is less than that of a neutron, free neutrons show beta decay. Free neutrons decay into protons, electrons and antineutrino.
Also Read:
| Related Articles | ||
|---|---|---|
| Group 15 Elements | Group 16 Elements | Group 18 Elements |
| Solutions | Types of Solutions | Solubility |
Applications of Neutrons
[Click Here for Sample Questions]
Some of the important applications of neutrons are given below:
- Neutrons are important for performing several nuclear reactions.
- Production of nuclear reactors and nuclear weapons requires the knowledge of neutrons.
- Neutrons are used in Neutron Activation Analysis (NAA) and Prompt Gamma Neutron Activation Analysis (PGNAA).
- Neutron emitters are used for the detection of light nuclei.
- Neutron radiations are used in the process of neutron scattering.
Read More: Orbitals
Things to Remember
- Neutrons are the neutral subatomic particles present in the nucleus of an atom.
- Protons and neutrons together form the nucleus of an atom and they are bound together by the strong nuclear force.
- A neutron consists of two down quarks and one up quark.
- The mass of a neutron is estimated to be 1.674923 × 10-27 kg and can be calculated by subtracting the mass of the proton from the mass of the deuteron.
- Neutrons were discovered by James Chadwick in 1932.
- Neutrons play an important role in performing several nuclear reactions.
Sample Questions
Ques. Who discovered Neutrons? Briefly explain the experiment conducted by him. (3 marks)
Ans. Neutrons were discovered by James Chadwick in 1932. He performed a series of experiments in order to discover neutrons.
Firstly, he emitted alpha radiation on a beryllium sheet from a polonium source. As a result, penetrating radiation was produced. To study the nature of this penetrating radiation, he made them fall on paraffin wax. As soon as the radiation fell on the paraffin wax, high energy protons were released. These protons were examined in ionisation chambers and radiation was also made to interact with other gaseous atoms. With all the results of his experiment, he concluded that this radiation consists of uncharged particles. James Chadwick later coined the term ‘neutrons’ for these uncharged particles.
Ques. What is Beta-decay? (3 marks)
Ans. β-decay is a type of radioactive decay occurring inside the nucleus. It is a process by which a neutron is converted into a proton or vice-versa. This happens because the mass of a neutron is slightly greater than that of a proton and free neutrons are unstable. During β-decay, a neutron is converted into proton, electron and antineutrino.
β-decay is of two types:
- Beta-Minus Decay - In this type, a neutron is converted to a proton. Alongwith the proton, an electron and antineutrino is also released.
- Beta-Plus Decay - In this type, a proton is converted to a neutron. A positron and neutrino are being released along with neutrons during the decay.
Beta-Minus decay results in the increase of the atomic number of an atom due to the production of a proton. Whereas, beta-plus decay leads to a decrease in the atomic number.
Ques. What is the difference between Isotopes and Isobars? (5 marks)
Ans. The main differences between isotopes and isobars are as follows:
| Isotopes | Isobars |
|---|---|
| Isotopes are the different atoms of the same element. | Isobars are the atoms of different elements. |
| They have the same atomic numbers. | They have different atomic numbers. |
| They have different atomic masses. | They have the same atomic masses. |
| Their chemical properties are similar. | Their chemical properties are different from each other. |
| Their physical properties are different. | Their physical properties are similar. |
Ques. What is the difference between Atomic Number and Atomic Mass? (3 marks)
Ans. The main differences between atomic number and mass number are:
| Atomic Number | Atomic Mass |
|---|---|
| Atomic number of an atom is equal to the number of protons present in the nucleus. | Atomic mass is the sum of the number of protons and number of neutrons present in the nucleus of an atom. |
| It is denoted by Z. | It is denoted by A. |
| Isotopes have the same atomic numbers but different atomic mass. | Isobars have the same atomic mass but different atomic numbers. |
| The atomic number of an atom does not depend upon the number of neutrons. | Atomic mass of an atom depends upon the number of neutrons. |
Ques. Calculate the mass and charge of one mole of electrons. (3 marks) [NCERT]
Ans. One mole of electrons = 6.022 × 1023 electrons
Mass of one electron = 9.1 × 10-31 kg
Mass of 6.022 × 1023 electrons = (9.1 × 10-31 kg) × (6.022 × 1023)
= 5.48 × 10-7 kg
Charge on one electron = 1.602 × 10-19 coulomb
Charge on one mole of electrons = 1.602 × 10-19 × 6.022 × 1023
= 9.65 × 104 coulombs
Ques. Calculate the number of protons and neutrons in the following nuclei (3 marks)
Ans. i. Atomic mass of carbon-13 = 13
Atomic number = number of protons = 6
Number of neutrons = atomic mass - number of protons
= 13 - 6
= 7
- Atomic number = number of protons = 8
Number of neutrons = atomic mass - number of protons
= 16 - 8
= 8
- Atomic number = number of protons = 12
Number of neutrons = atomic mass - number of protons
= 24 - 12
= 12
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Also Check:



Comments