Collegedunia Team Content Curator
Content Curator
Hund’s rule of maximum multiplicity deals with the filling of electrons in the subshell of atoms. It helps in determining the overall electronic configuration by removing the anomalies related to the same energy orbitals in a subshell. Electrons filling in the orbitals of the same subshell follow the guidelines as stated by Hund’s rule.
| Table of Content |
Keyterms: Electron, atoms, subshell, orbitals, Energy level, Aufbau rule, Pauli Exclusion rule, atomic spectra
Read More: Tollen’s Test
Hund’s Rule
[Click Here for Sample Questions]
Hund’s Rule of maximum multiplicity provides a method for arranging electrons in orbitals of a subshell. The Aufbau rule works according to the energy level of the subshells and determines the lowest energy subshell to be filled first. Each subshell (s,p,d,f) has a different number of orbitals. Electrons filling in the orbitals of the same subshell follows the guidelines as stated by Hund’s rule.
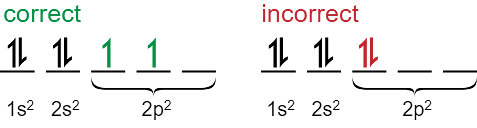
Hund’s Rule
The rule includes two basic parameters.
- Before completely filling a subshell, every orbital in that subshell must be singly filled.
- For maximum multiplicity, all electrons are assigned the same spin in orbitals with single occupancy.
Also Read:
Explanation Of Hund’s Rule
[Click Here for Previous Year Questions]
Each atom has subshells and a subshell can have a different number of orbitals. The Aufbau principle determines that the lowest energy orbital gets filled first. But an anomaly arises in the case of orbitals of the same subshell. The orbitals of the same subshell have the same energy. Hund’s rule comes into play here.
The following diagram illustrates how the 2p orbitals get filled up subsequently:
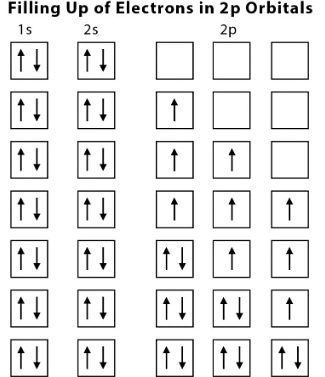
Filling up of Orbitals
According to Hund’s rule of maximum multiplicity, all electrons while entering a subshell, fill up the orbitals of the subshell singly i.e. unpaired electrons occupy all the orbitals first. All such electrons are having the same spin so as to maximise the multiplicity of spin. According to the rule, the lowest energy term in the electronic configuration will be the one with the highest value of spin multiplicity.
Read More: Atomic Mass of Elements
Hund’s Rule And Electronic Configuration
[Click Here for Sample Questions]
Three basic laws govern the overall electronic configuration of atoms of the periodic table: The Aufbau principle, The Hund’s rule and the Pauli Exclusion principle.
- Aufbau rule decides that the orbitals having the lowest energy shall be filled first.
- Pauli exclusion rule determines that no two electrons in an orbital can have the same spin.
- Hund’s rule focuses on the electronic configuration in the case of orbitals of the same subshell i.e. the degenerate orbitals. Let us take a few examples:
p-subshell

Filling of p subshell
Every p-subshell has 3 orbitals. First, the three orbitals get singly filled. Then the remaining electrons enter the orbitals to pair up. They follow the Pauli exclusion rule which maintains that inside an orbital electrons will have opposite spin.
d-subshell
Following Hund’s rule, the first five electrons in the five orbitals have the same spin. Then they pair up subsequently.

Filling of d subshell
The stability of any electronic configuration will increase if the electron-electron repulsion is minimised. So in the case of a subshell with singly filled degenerate orbitals, unpaired electrons are the farthest from each other. Hence stability is achieved through Hund’s rule.
Read More: Clemenson Reduction Reaction
Examples Of Hund’s Rule
[Click Here for Previous Year Questions]
Let us see how Hund’s rule affects the electronic configuration of degenerate orbitals of the following atoms:
Nitrogen atom
Electronic Configuration: 1s22s22p3

Nitrogen orbital filling
Here, the 2p subshell has three electrons. So accordingly before pairing all the orbitals will be filled with single electrons.
Oxygen atom
Electronic Configuration: 1s22s22p4

Oxygen orbital filling
Since an oxygen atom has one more electron than nitrogen, that electron will now pair up the electron in the first orbital of the p-subshell.
Carbon atom
Electronic configuration : 1s22s22p2

Carbon orbital filling
While filling up the 2p orbitals, 2 electrons occupy an orbital each with identical spin conforming to Hund’s rule.
Read More: Williamson Ether Synthesis
Applications of Hund’s Rule
[Click Here for Sample Questions]
- Hund’s rule has indispensable significance in quantum chemistry. It emphasises the stability of degenerate orbitals of the same subshell.
- Hund’s rule has uses in spectroscopy to generate the atomic spectra of elements.
- Hund’s rule helps in determining a stable electronic configuration by ensuring maximum multiplicity of spin.
Also Read:
Things To Remember
- Three basic laws govern the overall electronic configuration of atoms of the periodic table: The Aufbau principle, The Hund’s rule and the Pauli Exclusion principle.
- Maximum multiplicity of spin is obtained by the electrons filling the degenerate orbitals. This means that initially when all the orbitals are singly filled all the electrons will have identical spin.
- The Pauli exclusion rule determines that no two electrons in an orbital can have the same spin.
- Electronic configuration determines the orbital location of the electrons of an atom as well as the overall stability of the atom.
- According to the Aufbau rule, the orbital with the lowest energy gets filled first.
Previous Year Questions
- The stability of ferric ion is due to:...[BCECE 2006]
- Which one of the following represents the correct ratio of the energy of electron in ground state of H atom..[UPSEE 2011]
- An electron having spin quantum number, s = -1/2 and magnetic quantum number, m = + 3 can be present in...[COMEDK UGET 2012]
- The atomic number of the element with highest ionization energy among the following is...[COMEDK UGET 2012]
- The outer electronic configuration s2p5 is possessed by….[COMEDK UGET 2010]
- Which of the following pairs is isoelectronic?….[COMEDK UGET 2010]
- How many photons are emitted by the bulb per second?..[COMEDK UGET 2014]
- An orbital with n = 3, l = 1 is designated as….[COMEDK UGET 2014]
- In the following sets of ions, which one is not isoelectronic with the rest of the species?..[COMEDK UGET 2014]
- Energy associated with the first orbit of He+ is...[COMEDK UGET 2015]
- In photoelectric effect, the kinetic energy of the photoelectrons increases linearly with the...[JKCET 2010]
- For all gases, at any given pressure, the graph of volume vs temperature (in celsius) is a straight line. This graph is called...[COMEDK UGET 2014]
- What will be the longest wavelength of energy required to remove an electron from the third orbit?..[JKCET 2007]
- Which electronic level would allow the hydrogen atom to absorb a photon but not to emit a photon?..[JEE ADVANCED 1984]
- What is the maximum number of emission lines obtained when the excited electron of a hydrogen atom in n=5 drops to the ground state?..[JKCET 2010]
- Which of the following does not characterise X-rays ?..[JEE ADVANCED 1992]
Sample Questions
Ques. What is the purpose of electronic configuration? State Aufbau principle. (3 marks)
Ans. Valence electrons have the biggest role in an element’s chemical behaviour. An atom with unpaired electrons in its valence shell will be more unstable than the ones having all the electrons paired. This arrangement of electrons in the orbitals according to the energy of the subshells is called electronic configuration. The Aufbau rule, Hund’s multiplicity rule, and the Pauli exclusion rule help us determine a stable configuration.
Aufbau principle states that while filling up the electrons in the orbitals, the orbital with the least energy gets filled up first.
Ques. Write the electronic configuration of the Oxygen atom according to Aufbau and Hund’s rules. (3 marks)
Ans. Oxygen atom has 8 electrons. Hence the electronic configuration can be written as 1s22s22p4.

The first two electrons enter the 1s orbital, 2nd two electrons pair up in the 2s orbital. Now for the 2p orbitals, there are three orbitals. Each orbital will take two electrons. So first all the orbitals get one each. Now we have a single electron left. So the first orbital gets paired. This is the electronic configuration of an Oxygen atom conforming to Aufbau and Hund’s rules.
Ques. State a few applications of Hund’s rule. (2 marks)
Ans. Few applications of Hund’s rule are as follows:
- Hund’s rule has indispensable significance in quantum chemistry.It emphasises on the stability of degenerate orbitals of the same subshell.
- Hund’s rule has been used in spectroscopy to generate the atomic spectra of elements.
- Hund’s rule helps in determining a stable electronic configuration by ensuring maximum multiplicity of spin.
Ques. What is a multiplicity of spin? Explain it using an example. (3 marks)
Ans. Multiplicity of spin is given by the total number of spin orientations. Multiplicity of spin is calculated by the formula 2S+1. Hund’s rule says that the highest value of 2S+1 conforms to the lowest energy or the most stable configuration. The maximum value is attained only in the case of identical spins. Let us take the example of the 2p subshell. The 2p subshell has 3 orbitals. Let us fill 3 electrons in the three orbitals. The different arrangements possible are shown below:

The value of 2S+1 is maximum in the 1st and 4th cases since the electrons are having identical spins. So the spins add up resulting in a maximum spin.
Ques. Find out the spin multiplicity of the Oxygen atom. (2 marks)
Ans. The electronic configuration of the Oxygen atom is 1s22s22p4. Only one degenerate orbital of the 2p subshell gets paired up.

S = 1 and the multiplicity is 2S + 1 = 3.
Ques. Find the spin multiplicity of the Nitrogen atom. (2 marks)
Ans. Nitrogen has the electronic configuration of 1s22s22p3. Hence all the degenerate orbitals of the 2p subshell are half-filled.

So, spin = ½ +½ +½ = 3/2
And multiplicity = 2S+1 = 2(3/2)+1 = 4.
Ques. What subshells are possible in n=3 energy level? How many orbitals are possible at this level? (2 marks)
Ans. We know that the values of the quantum number l determine the number of subshells.
For n=3, l has the values: 0,1 and 2. So three subshells s, p and d are possible at n=3 energy level.
For n=3 energy level we have three subshells- s,p and d subshells. We know that the subshell has 1 orbital. p has 3 orbitals and d subshell has 5 orbitals. So the total number of orbitals at n=3 energy level is 9.
Ques.(a)If the quantum number l has a value of 2 then what can be the permitted values of ml?
(b)For an orbital with n=3, what are the different possible values of l?
(c) For l=3, what are the possible values of ml? (3 marks)
Ans.
- For l=2, the permitted values of ml are = -2 , -1 , 0 , +1 , +2 .
- For n=3, the possible values of l are = 0,1 and 2.
- For l=3 , the possible values of ml are = -3 , -2 , -1 , 0 , +1 , +2 , +3 .
Ques. Write the electronic configurations Fe2+ and find the total number of unpaired electrons in its ground state. (2 marks)
Ans. Fe2+ The electronic configuration of Fe is 1s22s22p63s23p63d64s2 .
For Fe2+ 2 electrons are removed from the 4s orbital so the resultant configuration is :
1s22s22p63s23p63d6 . All orbitals are completely filled except the 3d orbitals. We can write the arrangement of the 3d orbitals as follows
![]()
As is clear, the total number of unpaired electrons = 4.
Ques. Write the electronic configuration of Cl- ion. Also, find the total number of unpaired electrons in its ground state. (2 marks)
Ans. The electronic configuration of Clis 1s22s22p63s23p5 . Since Cl- has one electron extra. So its p subshell will be fully paired. Hence the electronic configuration for Cl- ion will be
1s22s22p63s23p6
So the total number of unpaired electrons is zero.
Do Check Out:



Comments