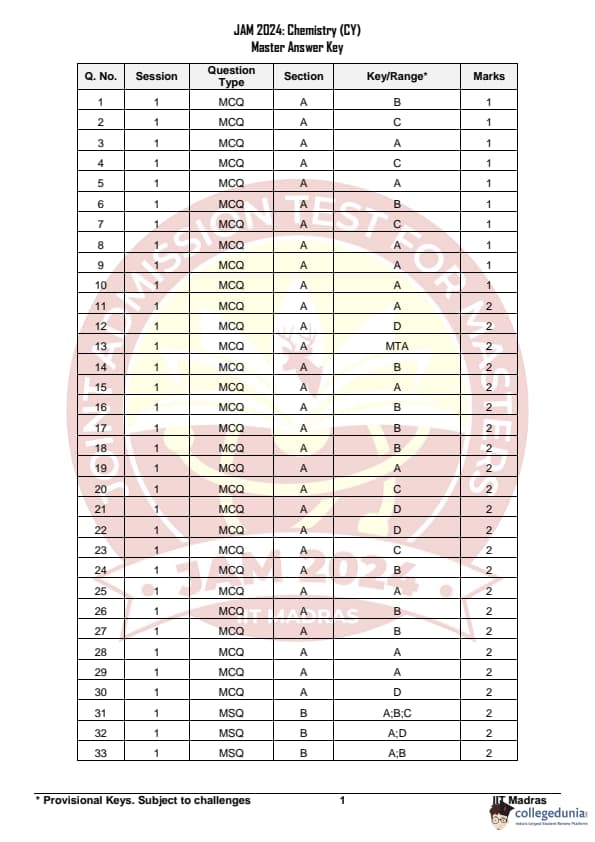
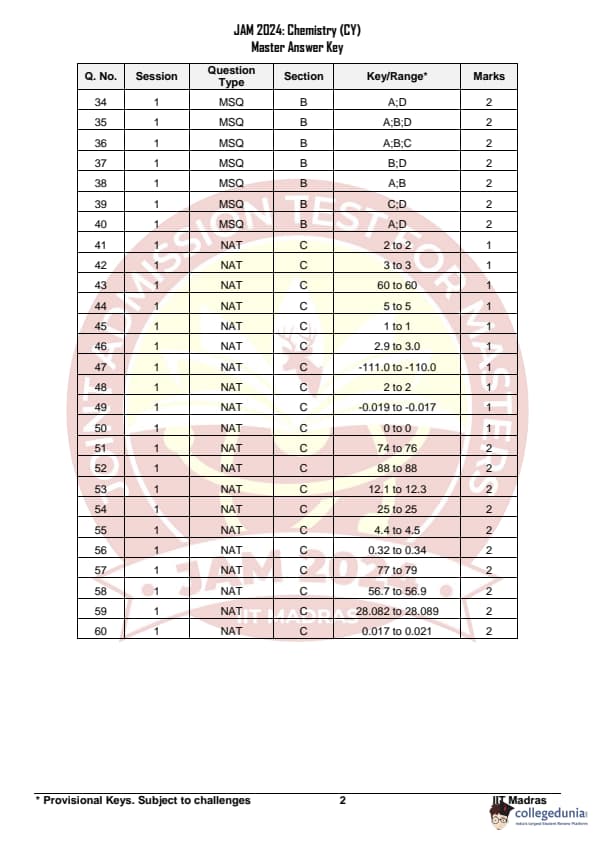
IIT JAM 2024 Chemistry (CY) Question Paper with Answer Key pdf is available for download. IIT JAM 2024 CY exam was conducted by IIT Madras in shift 1 on February 11, 2024. In terms of difficulty level, IIT JAM 2024 Chemistry (CY) paper was of easy to moderate. IIT JAM 2024 question paper for CY comprised a total of 60 questions.
IIT JAM 2024 Chemistry (CY) Question Paper with Solution PDFs
| IIT JAM 2024 Chemistry Question Paper PDF | Check Solutions |
IIT JAM 2024 Chemistry Questions with Solutions
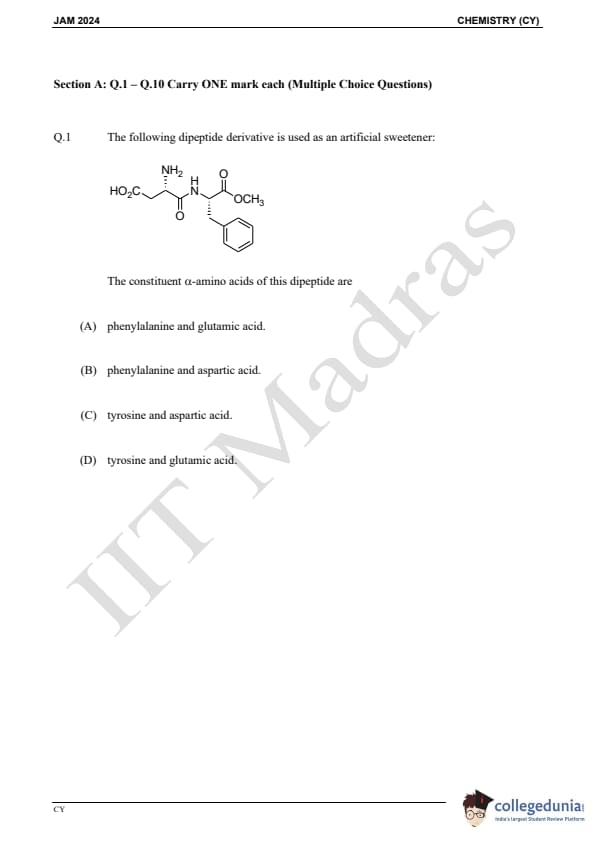
Question 1:
The following dipeptide derivative is used as an artificial sweetener:

The constituent α-amino acids of this dipeptide are:
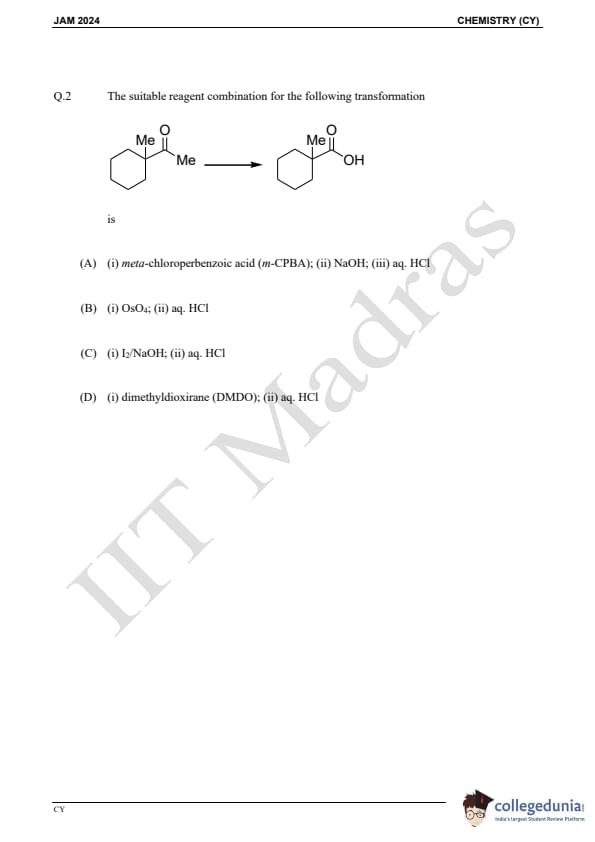
Question 2:
The suitable reagent combination for the following transformation is:

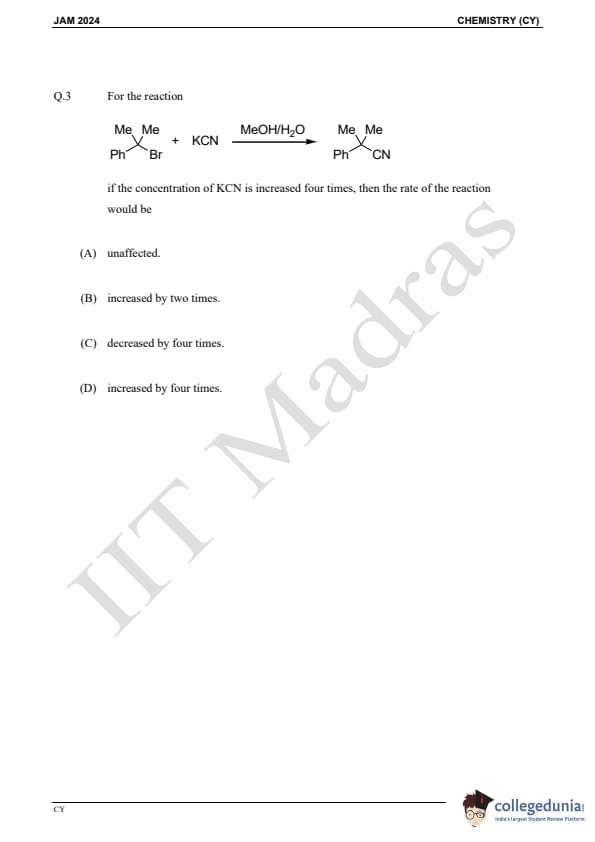
Question 3:
For the reaction:

If the concentration of KCN is increased four times, then the rate of the reaction would be:
Question 4:
Consider the wavefunction ψ(x) = N[exp(ikx) + exp(-ikx)]. The complex conjugate ψ*(x) is:
Question 5:
Wavelength of X-rays used in a diffraction experiment is 1.54 Å. X-rays are diffracted from a set of planes with an interplanar spacing of 1.54 Å. Then the angle θ (in degrees) corresponding to the first-order Bragg diffraction is:
Question 6:
Identify the reaction for which, at equilibrium, a change in the volume of the closed reaction vessel at a constant temperature will not affect the extent of the reaction.
Question 7:
Among [Ti(H2O)6]3+, [NiCl4]2-, [CrO4]2-, and [Mn(H2O)6]2+, the complex that exhibits the largest molar absorptivity in the visible region of the electronic absorption spectrum is:
Question 8:
[Co(NH3)6(SO4)]Br and [Co(NH3)6]BrSO4 are examples of:
Question 9:
The pair of proteins having heme core is:
Question 10:
The shape of SCN- is:
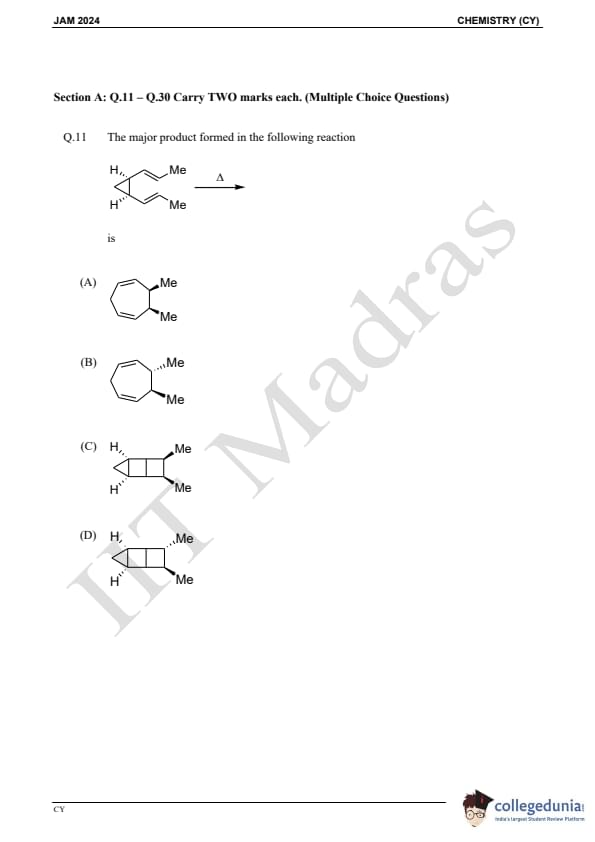
Question 11:
The major product formed in the following reaction is:
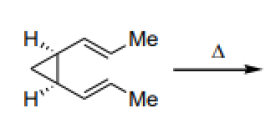
is
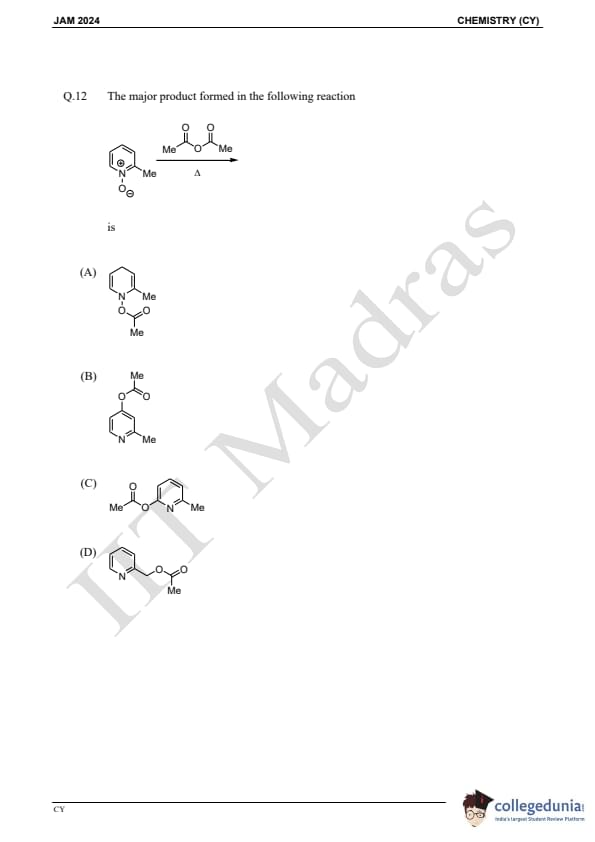
Question 12:
The major product formed in the following reaction is:
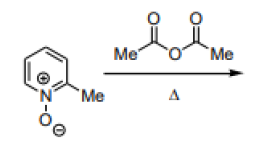
is
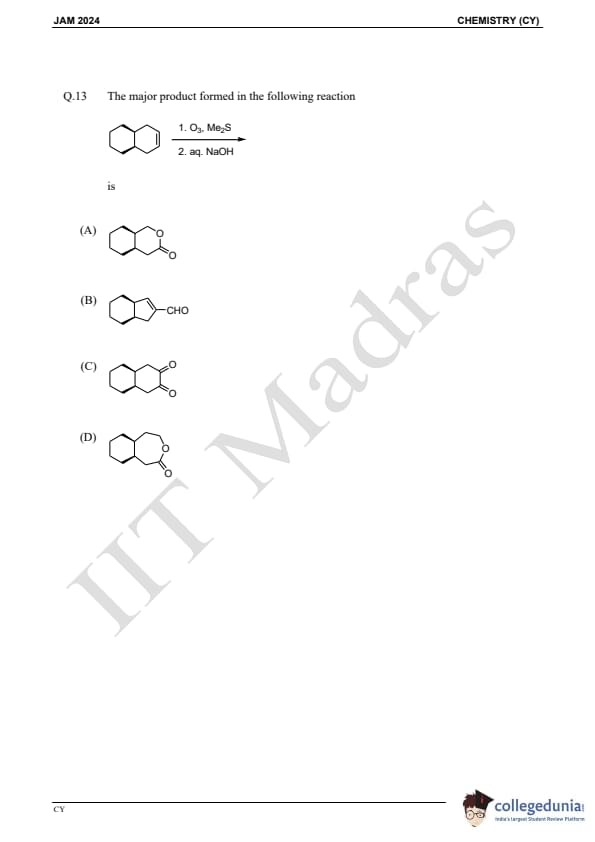
Question 13:
The major product formed in the following reaction is:

is
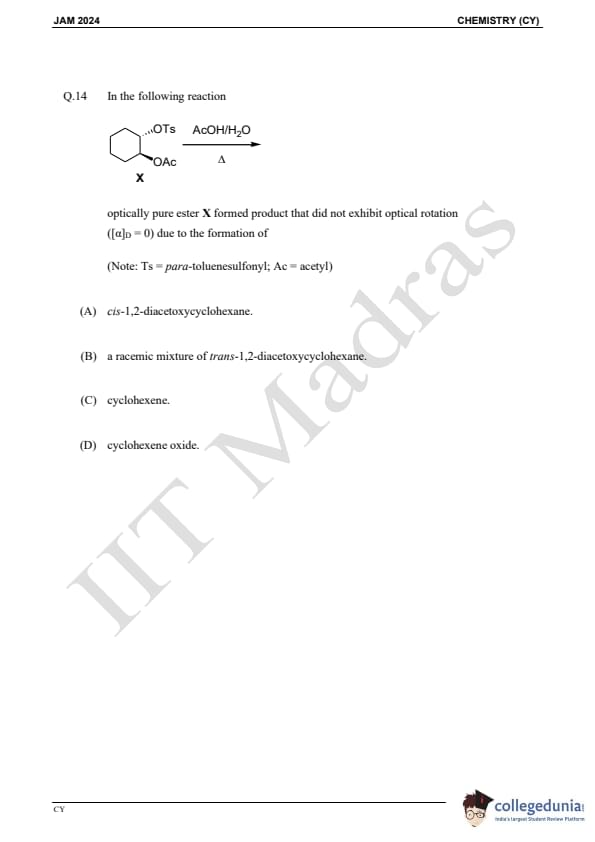
Question 14:
In the following reaction:

optically pure ester X formed a product that did not exhibit optical rotation ([α]D= 0) due to the formation of:
(Note: Ts = para-toluenesulfonyl; Ac = acetyl)
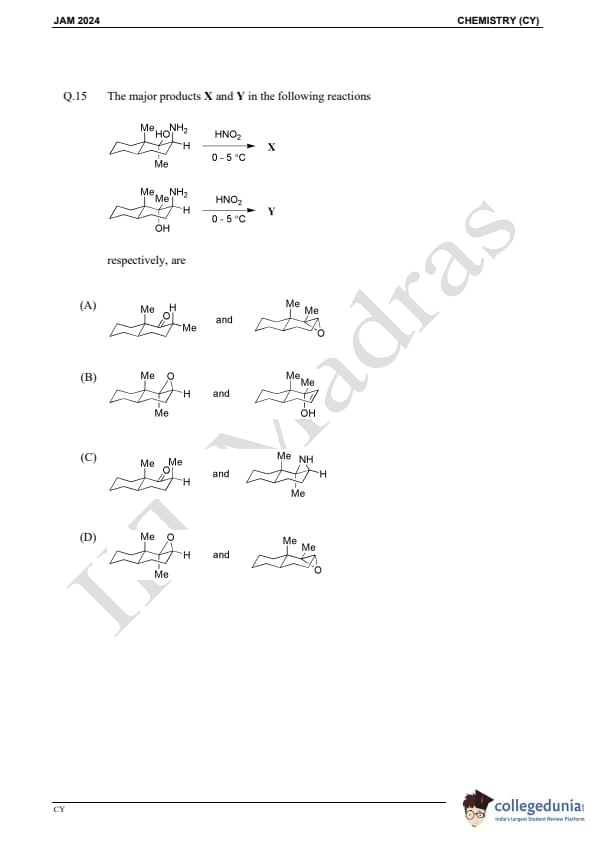
Question 15:
The major products X and Y in the following reactions respectively, are:
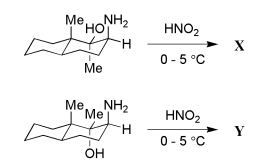
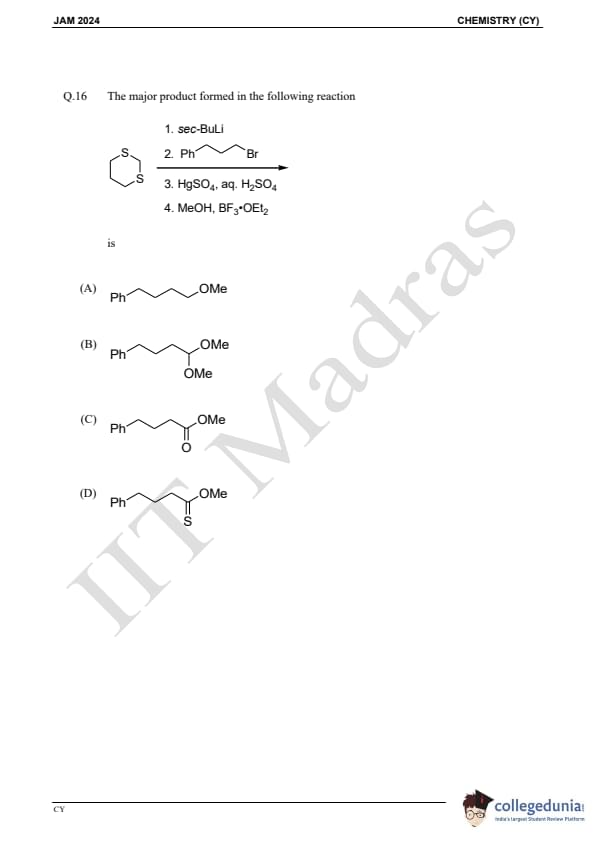
Question 16:
The major product formed in the following reaction is:
is
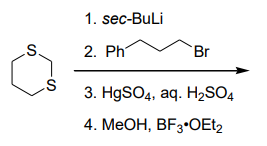
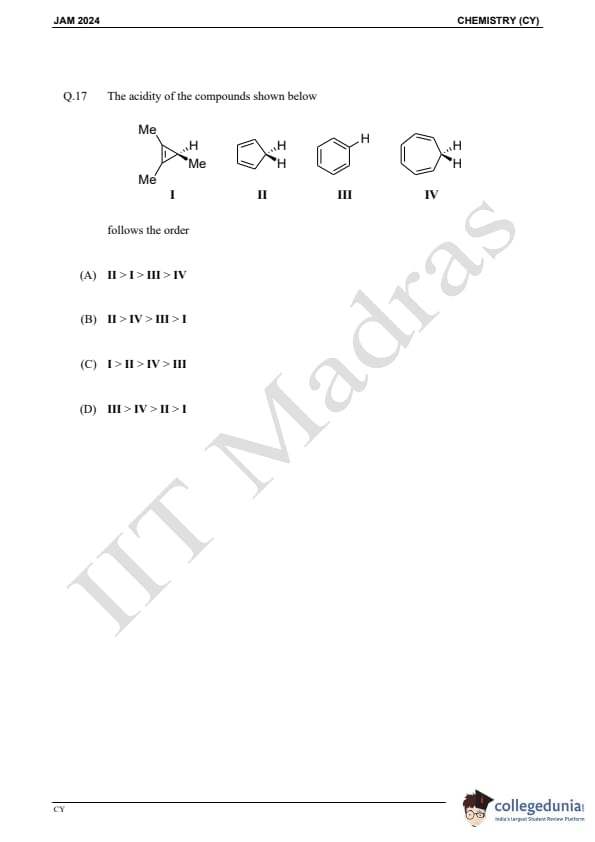
Question 17:
The acidity of the compounds shown below follows the order:
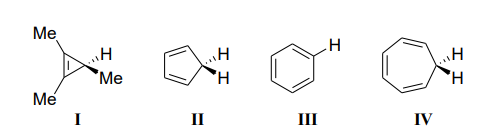
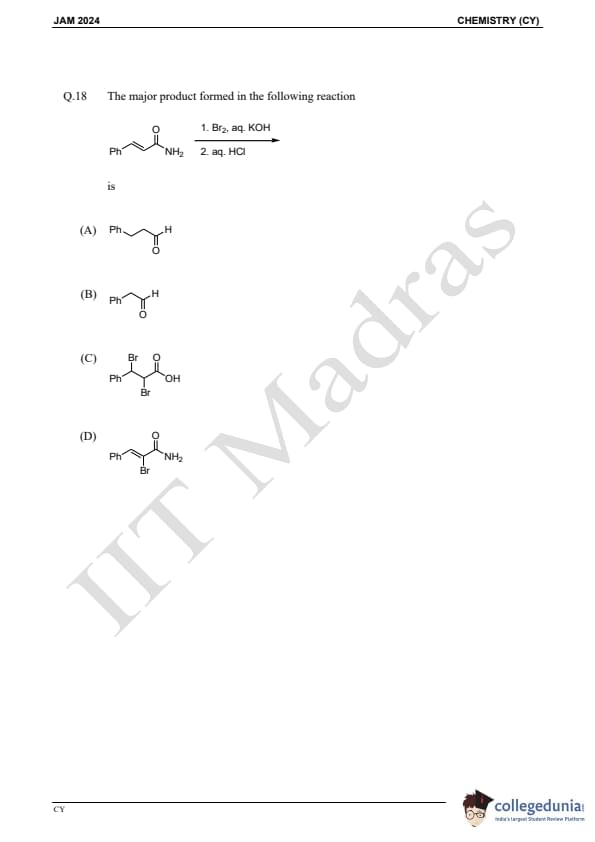
Question 18:
The major product formed in the following reaction is:
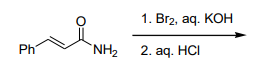
is
Question 19:
The ratio of osmotic pressures of aqueous solutions of 0.01 M BaCl2 to 0.005M NaCl is:
Question 20:
In the cell reaction P+(aq) + Q(s) → P(s) +Q+(aq) the EMF of the cell, Ecell, is zero. The standard EMF of the cell, Eocell is Given: Activities of all solids are unity. Activity of P+(aq) is 2 M. Activity of Q+(aq) is 1 M. R - universal gas constant; T -temperature; F - Faraday constant)
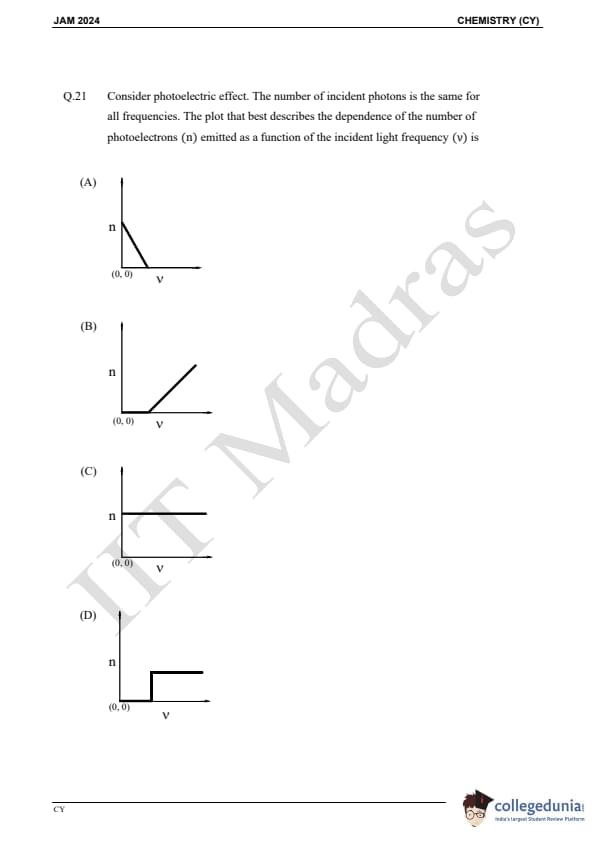
Question 21:
Consider photoelectric effect. The number of incident photons is the same for all frequencies. The plot that best describes the dependence of the number of photoelectrons n emitted as a function of the incident light frequency ν is:
Question 22:
If nitrogen and oxygen gases are at the same temperature, the correct statement according to the kinetic theory of gases is:
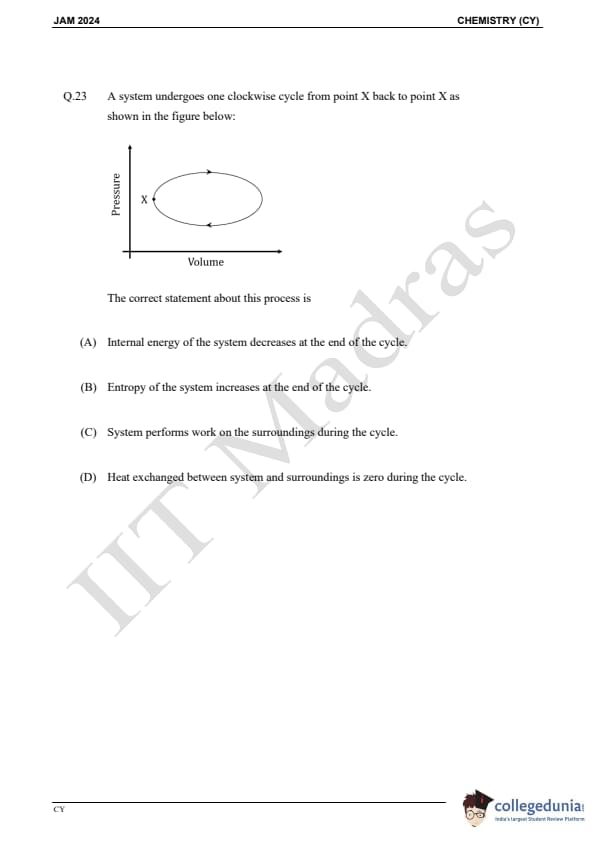
Question 23:
A system undergoes one clockwise cycle from point X back to point X as shown in the figure below:
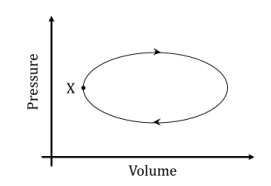
The correct statement about this process is:
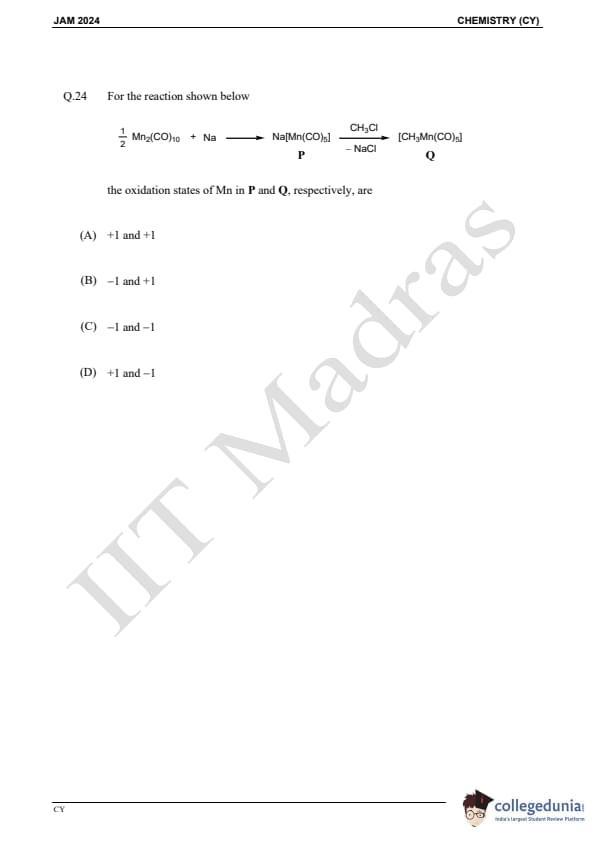
Question 24:
For the reaction below:
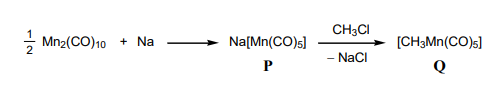
The oxidation states of Mn in P and Q, respectively, are:
Question 25:
The number and nature of d–d transition(s) in the case of Sc2+ in an octahedral crystal field, respectively, are:
Question 26:
The d-d transitions in [Mn(H2O)6]2+ and [Ti(H2O)4]3+, respectively, are:
Question 27:
A pair of isosteric compounds is:
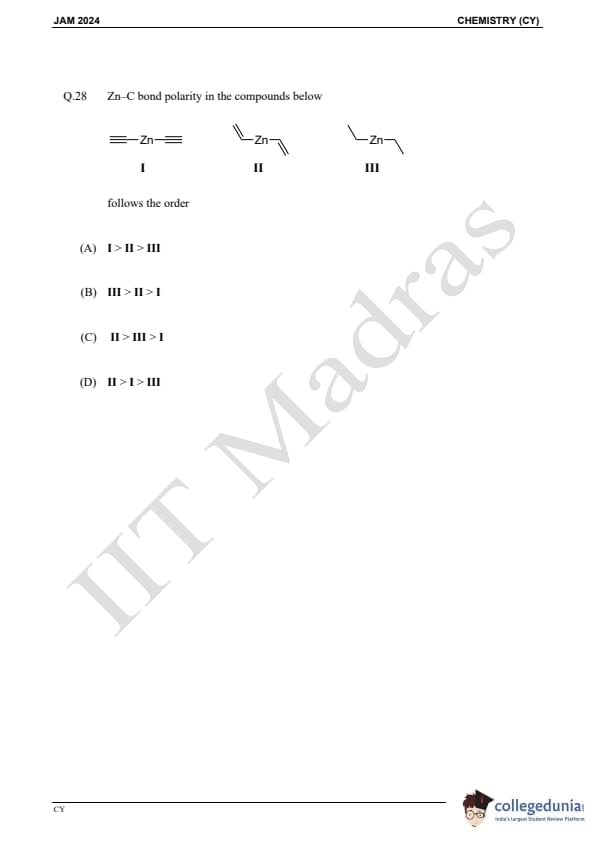
Question 28:
Zn–C bond polarity in the compounds below follows the order:
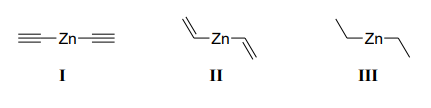
Question 29:
B2 and C2, respectively, are:
Question 30:
Mobility of ions Li+, Na+, K+, Ag+ in water at 298 K follows the order:
Question 31:
The suitable synthetic route(s) for the following transformation:
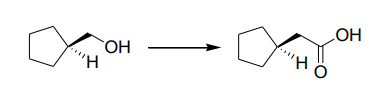
Cyclohexanol → Cyclohexanone
Question 32:
The compound(s) which on reaction with CH3MgBr followed by treatment with aqueous NH4Cl would produce 1-methyl-1-phenylethanol as the major product is/are:
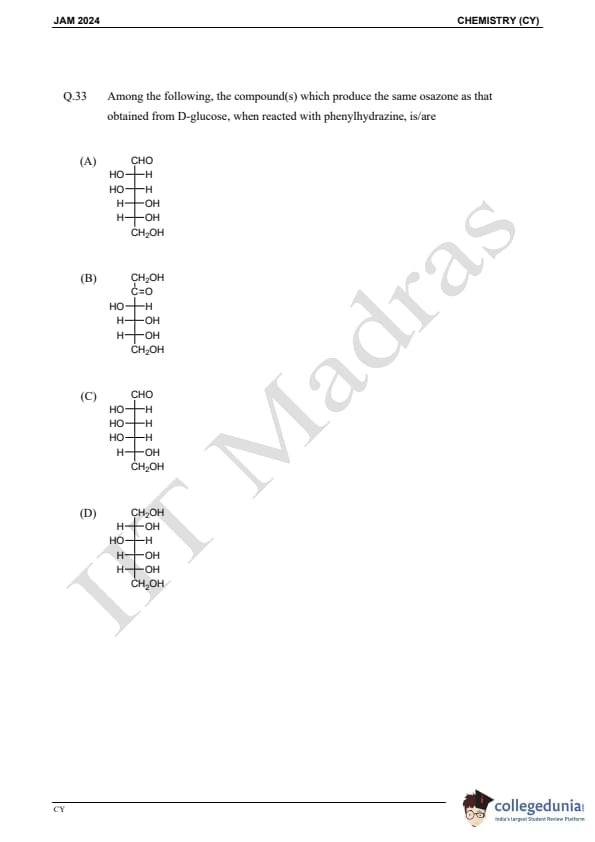
Question 33:
Among the following, the compound(s) which produce the same osazone as that obtained from D-glucose, when reacted with phenylhydrazine, is/are:
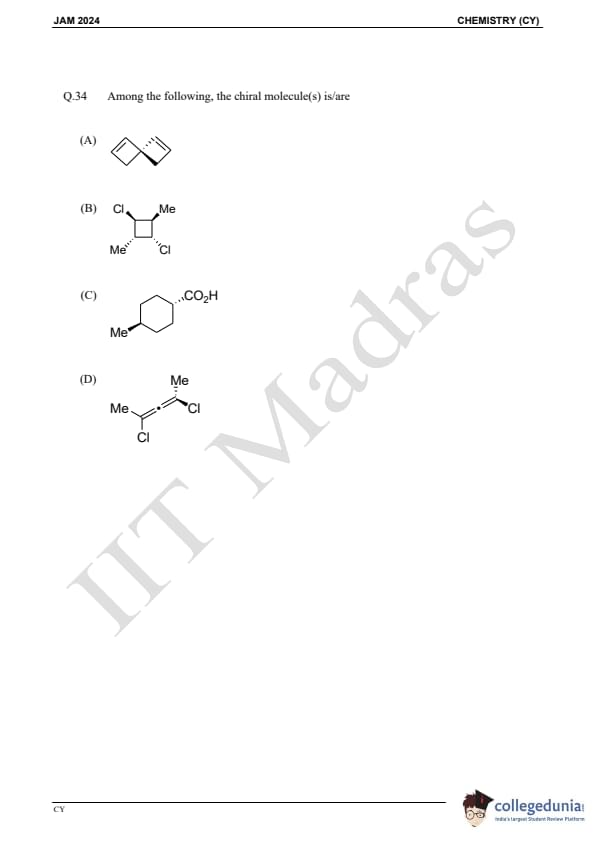
Question 34:
Among the following, the chiral molecule(s) is/are:
Question 35:
The correct assumption(s) required to derive Langmuir adsorption isotherm is/are:
Question 36:
For one mole of an ideal gas, the correct statement(s) is/are:
Question 37:
Consider the exothermic chemical reaction: O2(g)+2H2(g) = 2H2O(g) at equilibrium in a closed container. The correct statement(s) is/are:
Question 38:
Elements and their processes of extraction/purification are given. The correct pair(s) is/are:
Question 39:
The correct statement(s) about the ligand substitution/exchange reaction is/are:
Question 40:
The stretching frequency of CO in H3B·CO is:
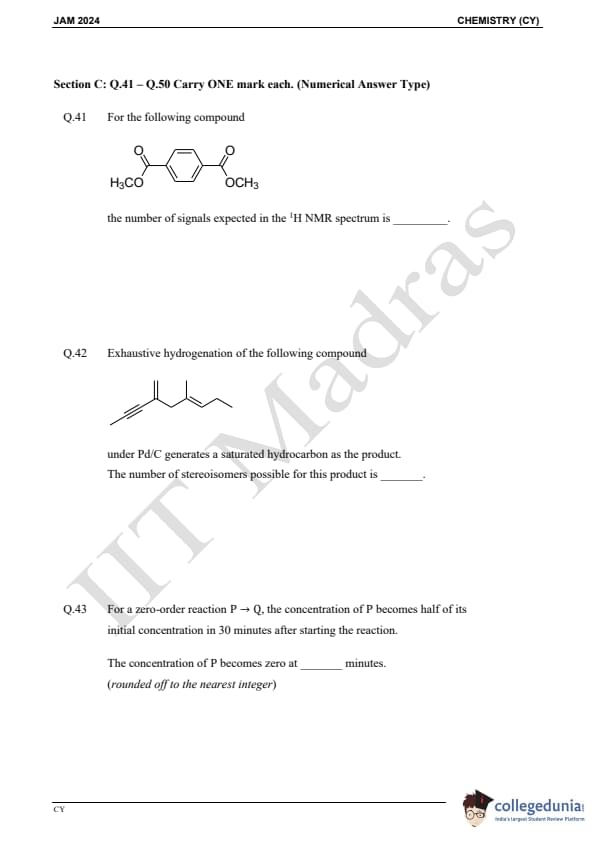
Question 41:
For the following compound, the number of signals expected in the 1H NMR spectrum is:
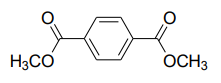
Question 42:
Exhaustive hydrogenation of the following compound under Pd/C generates a saturated hydrocarbon as the product. The number of stereoisomers possible for this product is:
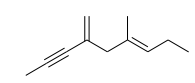
Question 43:
For a zero-order reaction P → Q, the concentration of P becomes half of its initial concentration in 30 minutes after starting the reaction. The concentration of P becomes zero at ______ minutes. (rounded off to the nearest integer)
Correct Answer:
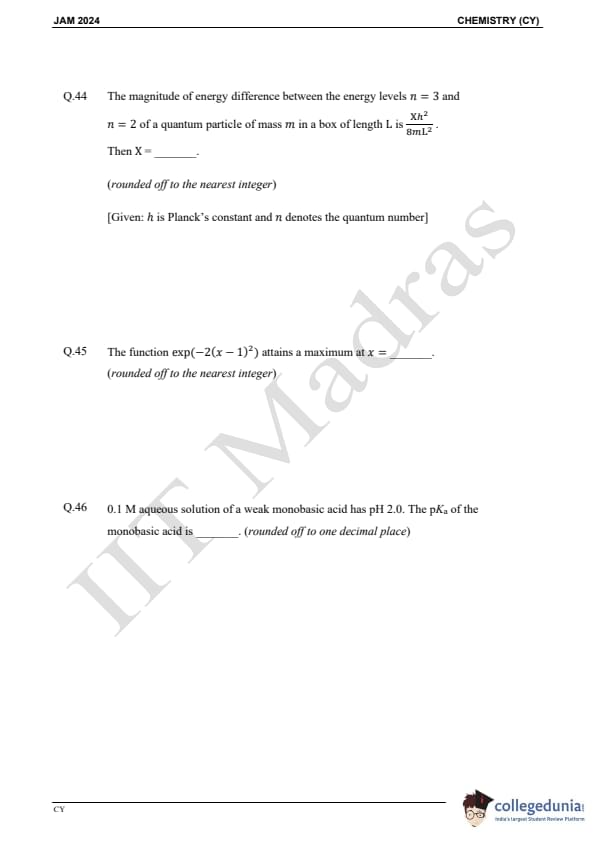
Question 44:
The magnitude of energy difference between the energy levels n = 3 and n = 2 of a quantum particle of mass m in a box of length L is
(Xh2)/(8mL2)
Then X = ________ (rounded off to the nearest integer)
Question 45:
The function exp(-2(x - 1)2) attains a maximum at x = ________ (rounded off to the nearest integer)
Question 46:
0.1 M aqueous solution of a weak monobasic acid has pH 2.0. The pKa of the monobasic acid is ________ (rounded off to one decimal place)
Question 47:
The enthalpy change for the reaction
C(g) + 1/2O2(g) → CO(g) is ________ kJ per mole of CO(g) produced.
(rounded off to one decimal place)
[Given:
C(g) + O2(g) → CO2(g), ΔHrxn = -393.5 kJ per mole of CO2(g) produced
CO2(g) → CO(g) + 1/2O2(g), ΔHrxn = 283.0 kJ per mole of CO(g) produced]
Question 48:
The N-O bond order in [NO]- is ________
Question 49:
The bond length of CO is 113 pm and its dipole moment (μ) is 0.1 D. The charge (in units of electronic charge) on carbon in the CO molecule including its sign is _________
Question 50:
A reaction of 10.50 g of 1,2-diphenylethane-1,2-dione with conc. NaOH followed by aqueous acidic work-up furnished 8.55 g of a carboxylic acid. The yield of the carboxylic acid in this reaction is _________%.
Question 51:
The specific rotation of an optically pure compound is +75.3 (c = 1.0 in CHCl3) at 20°C. A synthetic sample of the same compound showed a specific rotation of +66.3 (c = 1.0 in CHCl3) at 20°C. The enantiomeric excess (ee) of the synthetic sample is ________%
Question 52:
The specific rotation of an optically pure compound is +75.3 (c = 1.0 in CHCl3) at 20°C. A synthetic sample of the same compound showed a specific rotation of +66.3 (c = 1.0 in CHCl3) at 20°C. The enantiomeric excess (ee) of the synthetic sample is ________%
Question 53:
A salt QCl of a certain metal Q is electrolyzed to its elements. 40 g of metal Q is formed at an electrode. The volume of Cl2 formed at the other electrode at 1 atm pressure and 298 K is ________ litres.
Question 54:
If 1 M of a dye in water transmits 50% of incident light at 400 nm, then 2 M of the dye in water transmits ________% of the incident light at 400 nm.
Question 55:
A 1.0 L solution is prepared by dissolving 2.0 g of benzoic acid and 4.0 g of sodium benzoate in water. The pH of the resulting solution is ________ Given: Molar mass of benzoic acid is 122 g mol-1 Molar mass of sodium benzoate is 144 g mol-1 pKa of benzoic acid is 4.2)
Question 56:
The total vapour pressure of an ideal binary liquid mixture of benzene and toluene is 0.3 bar. The vapour pressure of pure benzene is 0.5 bar and that of toluene is 0.2 bar. The mole fraction of benzene in this mixture is _________.
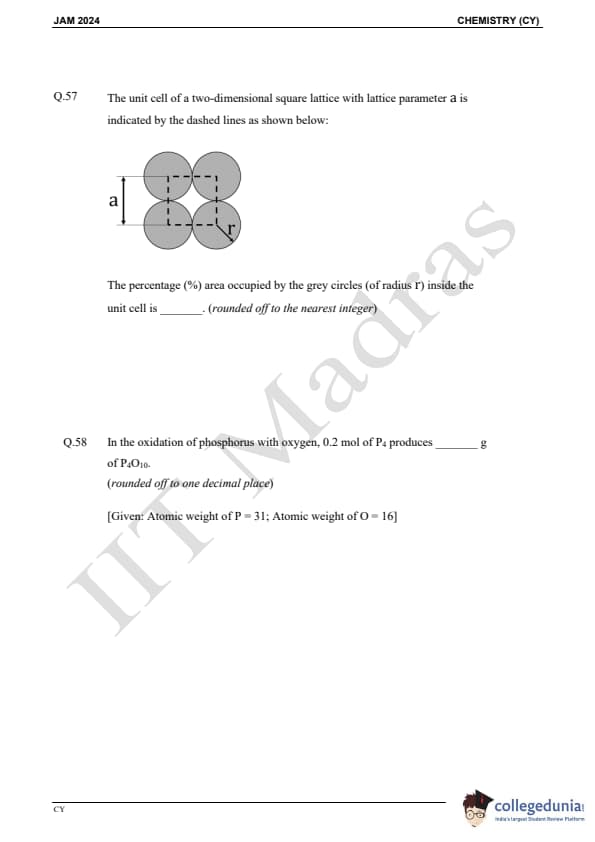
Question 57:
The unit cell of a two-dimensional square lattice with lattice parameter a is indicated by the dashed lines as shown below:

The percentage percent area occupied by the grey circles (of radius r) inside the unit cell is ___________.
Question 58:
In the oxidation of phosphorus with oxygen, 0.2 mol of P produces ________ g of PO. (Given: Atomic weight of P = 31; Atomic weight of O = 16)
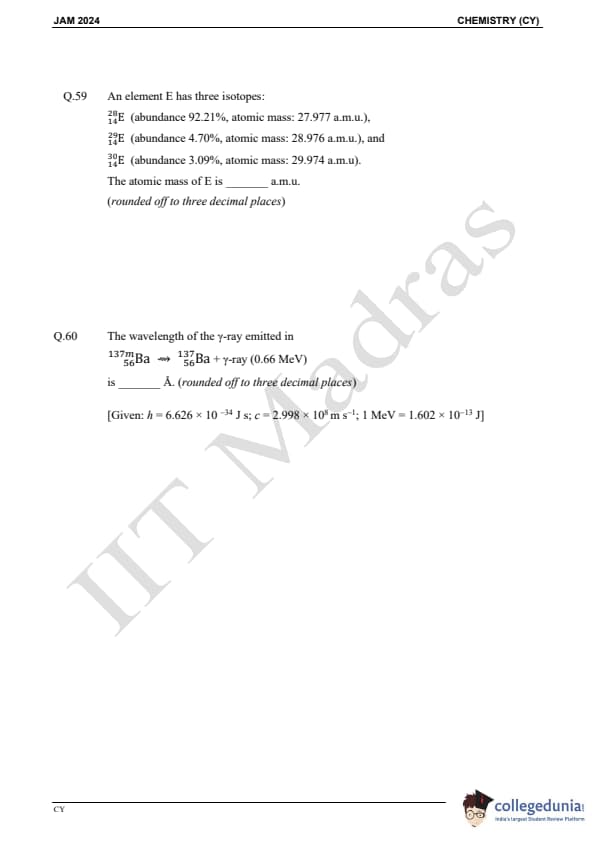
Question 59:
An element E has three isotopes: 28E (abundance 92.21%, atomic mass = 27.977 a.m.u.), 29E (abundance 4.70%, atomic mass = 28.976 a.m.u.), 30E (abundance 3.09%, atomic mass = 29.974 a.m.u.). The atomic mass of E is ________ a.m.u.
Question 60:
The wavelength of the γ-ray emitted in 13756Ba → 13756Ba + γ-ray (0.66 MeV) is ________ Å.



























IIT JAM Previous Year Question Papers
| IIT JAM 2023 Question Papers | IIT JAM 2022 Question Papers | IIT JAM 2021 Question Papers |
| IIT JAM 2020 Question Papers | IIT JAM 2019 Question Papers | IIT JAM 2018 Question Papers |






































Comments