IIT JAM 2024 Biotechnology (BT) Question Paper with Answer Key pdf is available for download. IIT JAM 2024 BT exam was conducted by IIT Madras in shift 2 on February 11, 2024. In terms of difficulty level, IIT JAM 2024 Biotechnology (BT) paper was of moderate level. IIT JAM 2024 question paper for BT comprised a total of 60 questions.
IIT JAM 2024 Biotechnology (BT) Question Paper with Solution PDFs
| IIT JAM 2024 Biotechnology Question Paper PDF | Check SolutionsCheck Solutions |
IIT JAM 2024 Biotechnology Questions with Solutions
Question 1:
Which one of the following is a simple tissue system in plants?
Question 2:
In DNA replication, the Okazaki fragments are joined by:
Question 3:
The most abundant type of RNA in a metabolically active mammalian cell is:
Question 4:
Which organelle in a eukaryotic cell is the site of the electron transport chain?
Question 5:
RNA is a polymer of:
Question 6:
Which one of the following is present in a bacterial cell?
Question 7:
Which color of light excites a natural GFP to emit green fluorescence?
Question 8:
Which one of the following hormones promotes fruit ripening?
Question 9:
Which one of the following has a catalytic RNA?
Question 10:
The number of significant figures in a reported measurement of 0.00361 is:
Question 11:
Match the terminology in Group I with the stimulus in Group II that generates growth response of plants.
Group I: P. Gravitropism Q. Phototropism R. Thigmotropism S. Chemotropism
Group II: 1. Light 2. Touch 3. Chemical 4. Gravity
Question 12:
The correct hierarchy of taxa in the Linnaean classification of eukaryotes is:
Question 13:
Which one of the following statements about polyploidy is correct?
Question 14:
Which one of the following hormones is a tyrosine derivative?
Question 15:
Which one of the following immunoglobulins crosses the human placenta?
Question 16:
Determine the correctness or otherwise of the following Assertion (A) and the Reason (R).
Assertion (A): The resolving power of a transmission electron microscope is higher than that of the light microscope.
Reason (R): The wavelength of electrons is shorter than that of visible light.
Question 17:
Match the morphology in Group I with the corresponding microorganism in Group II.
Group I: P. Coccus Q. Rod R. Comma S. Spiral
Group II: 1. Treponema 2. Bacillus 3. Neisseria 4. Vibrio
Question 18:
Which one of the following genetic crosses and their results indicates cytoplasmic inheritance?
Question 19:
Which of the following is NOT a characteristic morphological feature of apoptotic cells?
Question 20:
Competition between two populations in an ecosystem is:
Question 21:
Adenine constitutes 0.16 mole fraction in a given single-stranded DNA. What is the mole fraction of uracil in the resultant RNA, if this entire DNA fragment is transcribed?
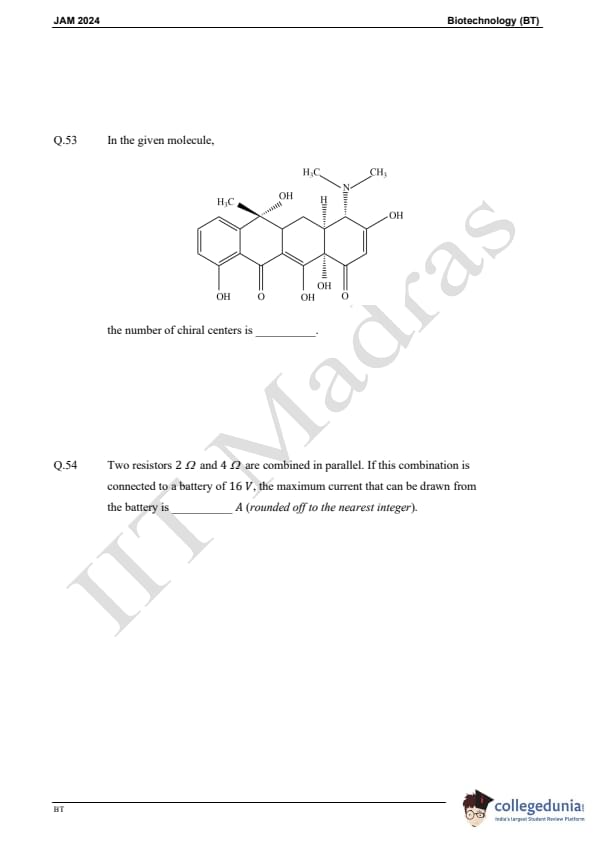
Question 22:
Which one of the following is NOT used as a component in subunit vaccines?
Question 23:
Metabolic acidosis is associated with decreased plasma level of:
Question 24:
Genes in two species that are derived from the same ancestral gene in their most recent common ancestor are called:
Question 25:
An object is placed 15 cm in front of a convex mirror, which has a radius of curvature 30 cm. Which one of the following is true of the image formed?
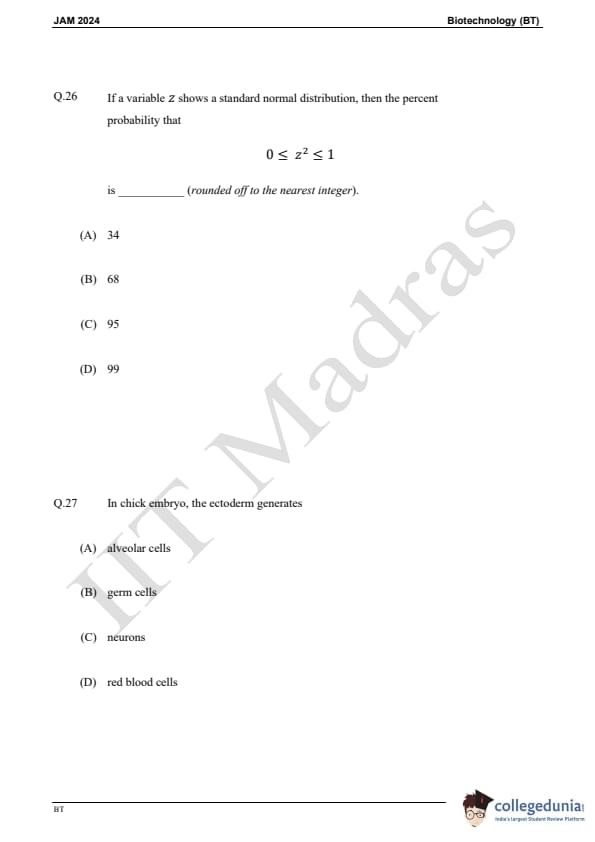
Question 26:
If a variable z shows a standard normal distribution, then the percent probability that 0 < z² < 1 is:
Question 27:
In the chick embryo, the ectoderm generates:
Question 28:
The boiling points of Iodomethane, Dibromomethane, Bromomethane, Chloromethane follow the order:
Question 29:
Chromosome duplication during the cell cycle occurs in:
Question 30:
Ionic character of the covalent bonds in the compounds Cl2, HCl, NaCl, NaF follows the order:
Question 31:
Which of the following is/are lateral meristems?
Question 32:
Which of the following statement(s) about Golden Rice is/are correct?
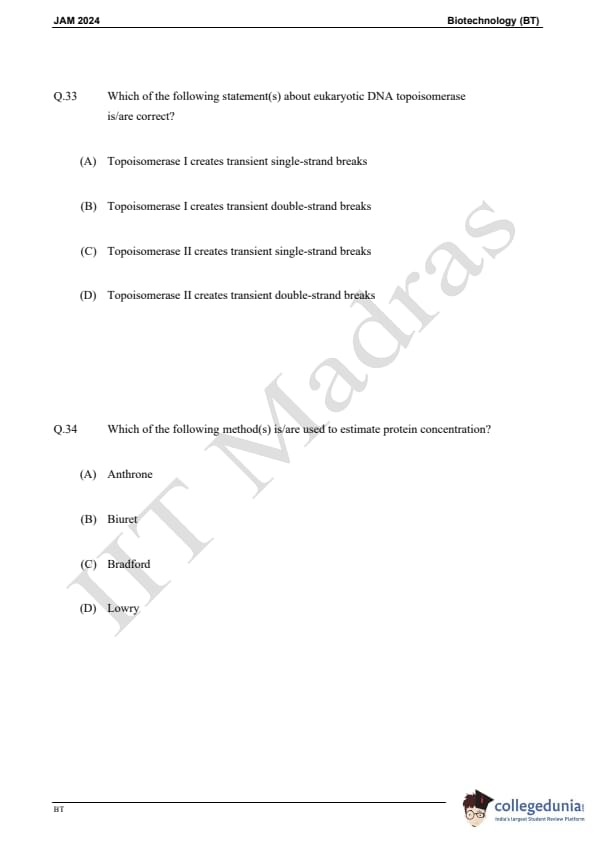
Question 33:
Which of the following statement(s) about eukaryotic DNA topoisomerase is/are correct?
Question 34:
Which of the following method(s) is/are used to estimate protein concentration?
Question 35:
Which of the following is/are example(s) of a lotic ecosystem?
Question 36:
Which of the following statement(s) about the effect of genetic drift is/are correct?
Question 37:
Which of the following technique(s) can be used to determine the three-dimensional structure of an organic compound?
Question 38:
Which of the following entity(ies) is/are found inside the intact nucleus of eukaryotic cells?
Question 39:
Which of the following is/are trace element(s)?
Question 40:
Which of the following is/are true about Retrovirus?
Question 41:
A wooden plant accumulates 10 mg/kg of 14C during its life span. A fossil of this plant was discovered and contains 2.5 mg/kg of 14C. The age of this fossil at the time of discovery is (use 5730 years as the half-life of 14C):
Question 42:
A cylinder contains 50 L of an ideal gas at a pressure of 50 atm. Assuming that the temperature remains unchanged, the volume of the gas at 1 atm is:
Question 43:
One molecule of the protein myoglobin contains one atom of iron. A myoglobin sample was found to contain 0.34% iron. The molecular weight of myoglobin is (use atomic mass of iron = 55.9 g/mol):
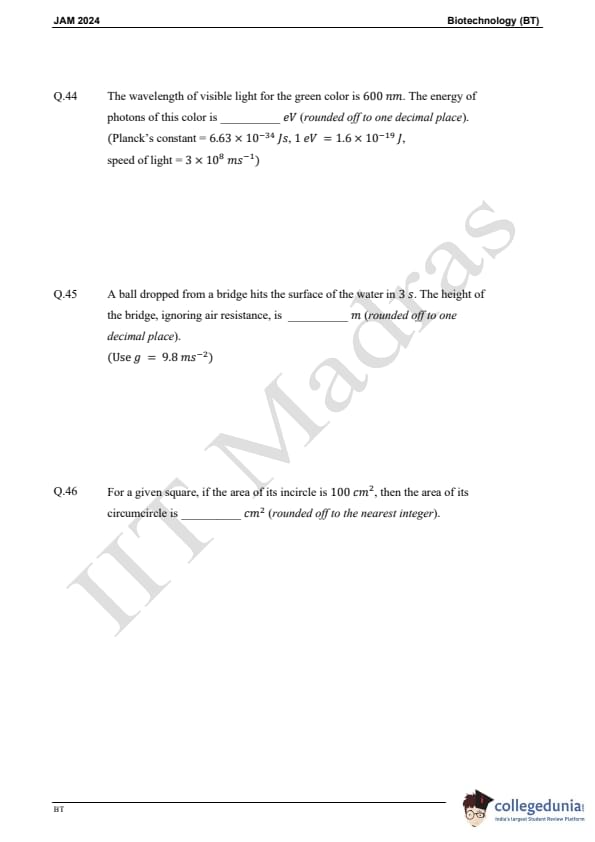
Question 44:
The wavelength of visible light for the green color is 600 nm. The energy of photons of this color is (Planck's constant h = 6.63 × 10-34 J.s, 1 eV = 1.6 × 10-19 J, speed of light c = 3 × 108 m/s):
Question 45:
A ball dropped from a bridge hits the surface of the water in 3 s. The height of the bridge, ignoring air resistance, is (use g = 9.8 m/s²):
Question 46:
For a given square, if the area of its incircle is 100 cm², then the area of its circumcircle is:
Question 47:
The number of peaks in the 1H NMR spectrum of methoxymethane (CH3OCH3) is:
Question 48:
The amount of agarose required to prepare 250 mL of 0.8% agarose gel is:
Question 49:
Three genes x, y, and z are located on a chromosome in a linear order. If the recombination frequencies between x and y is 0.15, and between y and z is 0.10, the expected frequency of double crossovers is:
Question 50:
A bacterial cell suspension contains 2 × 105 cells/mL. The volume of this suspension required to obtain 1.4 × 106 cells is:
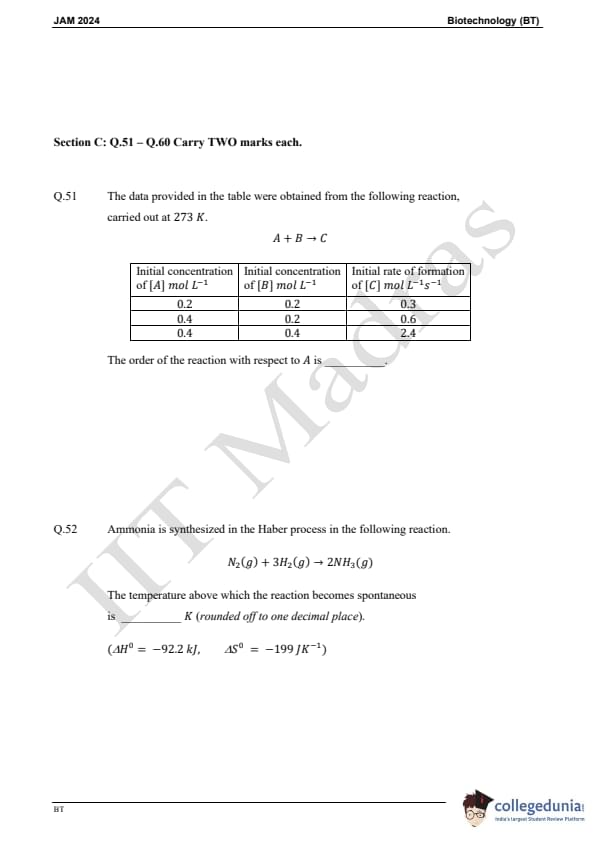
Question 51:
The data provided in the table were obtained from the following reaction, carried out at 273 K:
A + B → C
| Initial concentration of [A] | Initial concentration of [B] | Initial rate of formation of [C] |
|---|---|---|
| 0.2 mol/L | 0.2 mol/L | 0.3 mol/L/s |
| 0.4 mol/L | 0.2 mol/L | 0.6 mol/L/s |
| 0.4 mol/L | 0.4 mol/L | 2.4 mol/L/s |
The order of the reaction with respect to [A] is:
Question 52:
Ammonia is synthesized in the Haber process in the following reaction:
N2(g) + 3H2(g) → 2NH3(g)
The temperature above which the reaction becomes spontaneous is:
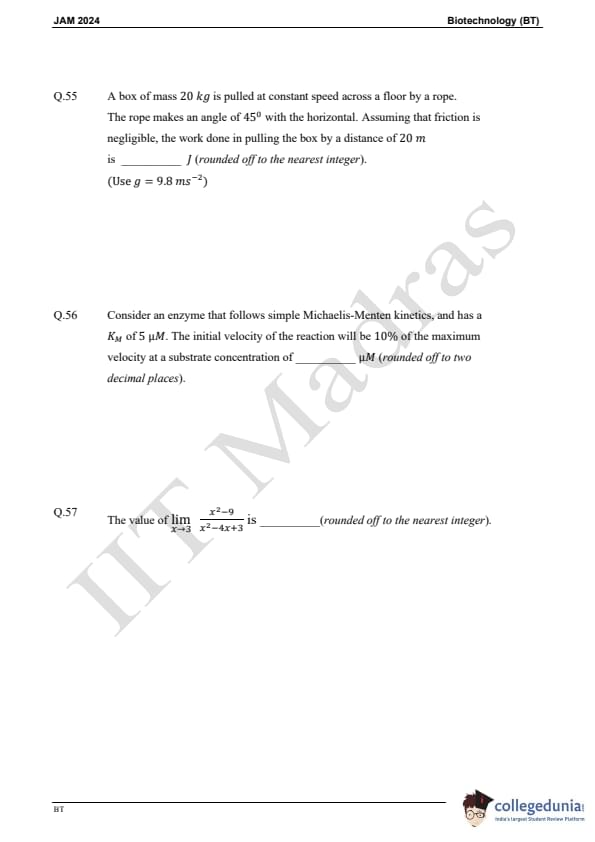
Question 54:
Two resistors of 2 Ω and 4 Ω are combined in parallel. If this combination is connected to a battery of 16 V, the maximum current that can be drawn from the battery is:
Question 55:
A box of mass 20 kg is pulled at constant speed across a floor by a rope. The rope makes an angle of 45° with the horizontal. Assuming that friction is negligible, the work done in pulling the box by a distance of 20 m is:
Question 56:
Consider an enzyme that follows simple Michaelis-Menten kinetics, and has a KM of 5 μM. The initial velocity of the reaction will be 10 percent of the maximum velocity at a substrate concentration of:
Question 57:
The value of: lim (x2 - 9) / (x2 - 4x + 3) as x approaches 3
Question 58:
A population of 1000 plants are in Hardy-Weinberg equilibrium. Two alleles R and r determine a particular trait in this population. If the number of plants with RR genotype is 640, Rr genotype is 320, and rr genotype is 40, the frequency of the r allele (in percentage) in this population is:
Question 59:
If a fair coin is tossed two times, the probability that the first or the second toss will be heads is:
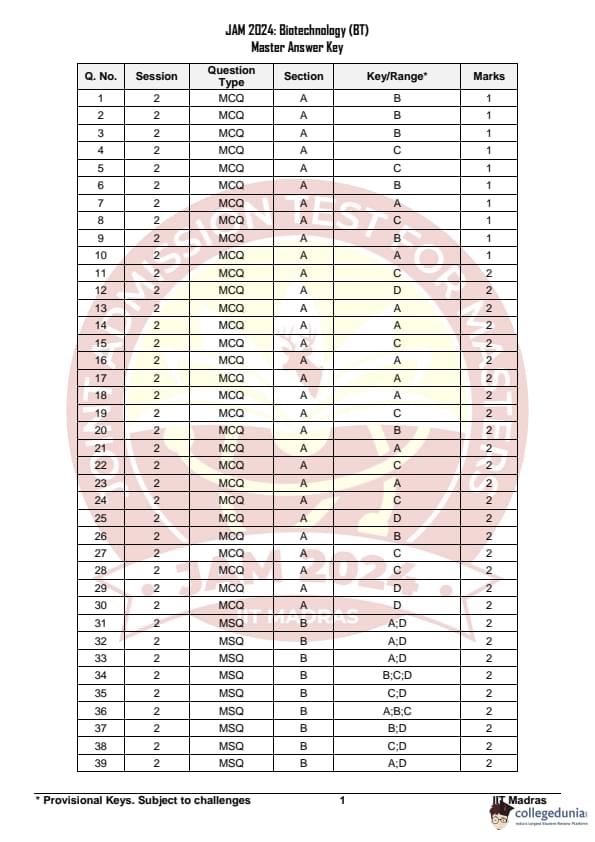
Question 60:
The number of bands that will be visible in the gel when exposed to UV light after complete digestion of a circular plasmid with EcoRI and XhoI is:
























IIT JAM Previous Year Question Papers
| IIT JAM 2023 Question Papers | IIT JAM 2022 Question Papers | IIT JAM 2021 Question Papers |
| IIT JAM 2020 Question Papers | IIT JAM 2019 Question Papers | IIT JAM 2018 Question Papers |




Comments