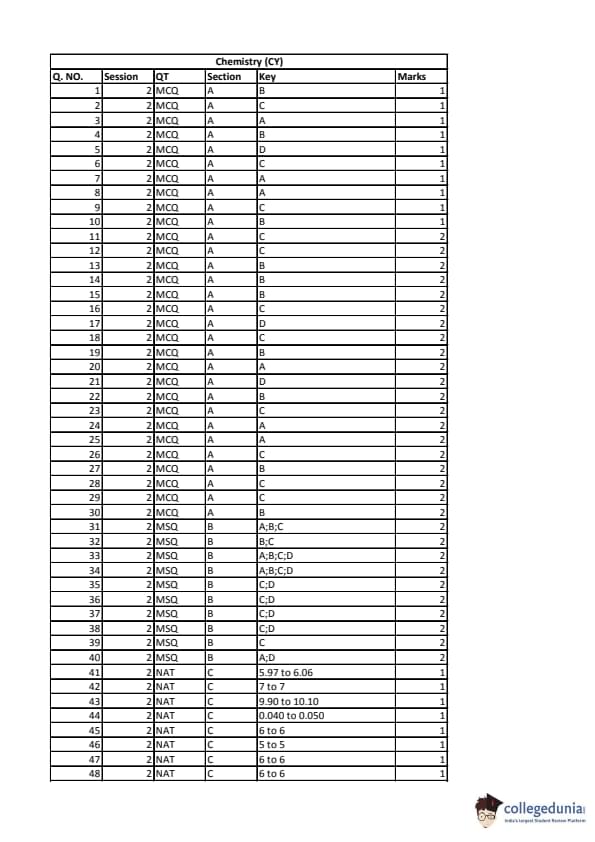
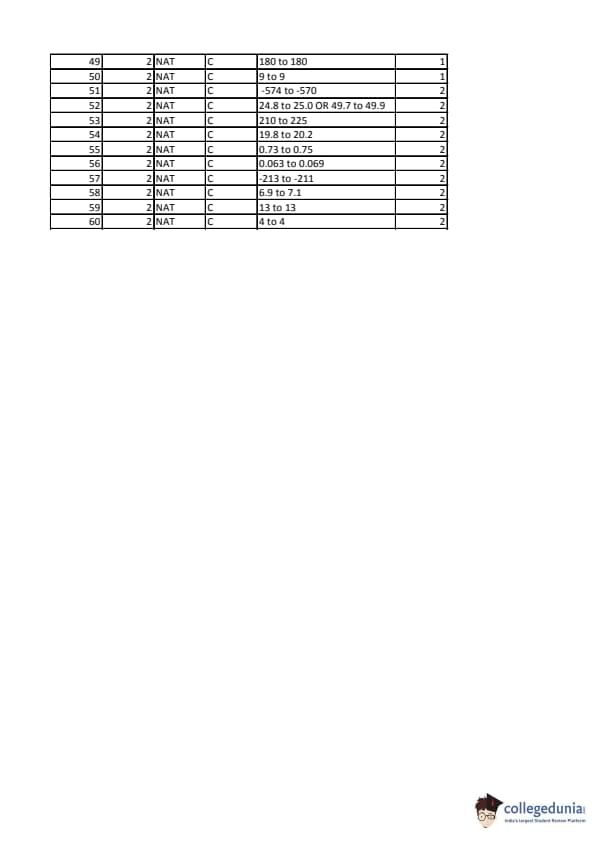
IIT JAM 2020 Chemistry (CY) Question paper with answer key pdf conducted on February 9 in Afternoon Session 2:30 PM to 5:30 PM is available for download. The exam was successfully organized by IIT Kanpur. In terms of difficulty level, IIT JAM was of Moderate level. The question paper comprised a total of 60 questions divided among 3 sections.
IIT JAM 2020 Chemistry (CY) Question Paper with Answer Key PDFs Afternoon Session
| IIT JAM 2020 Chemistry (CY) Question paper with answer key PDF | Download PDF | Check Solutions |
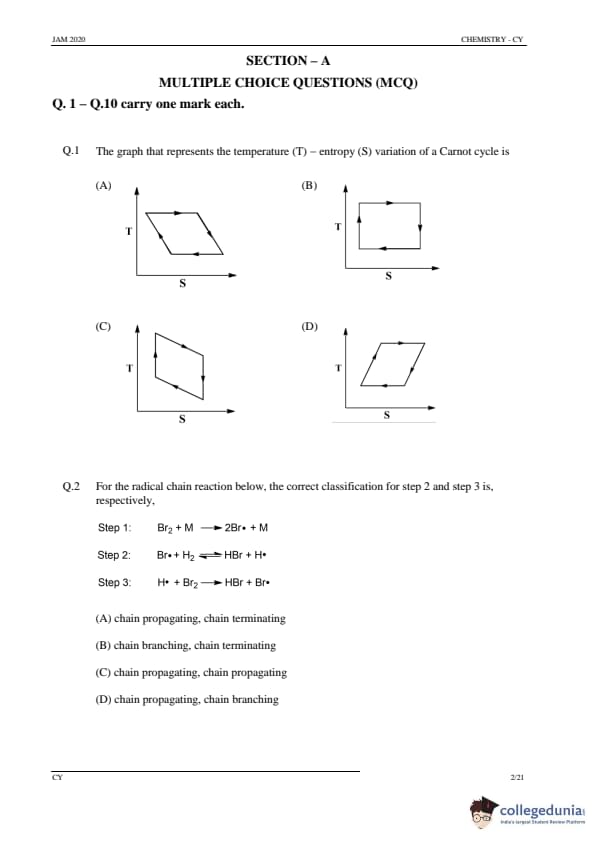
The graph that represents the temperature (T) – entropy (S) variation of a Carnot cycle is

View Solution
Step 1: Understanding the Carnot cycle.
The Carnot cycle consists of two isothermal processes (heat absorption and heat rejection) and two adiabatic processes. In a T-S diagram, these processes are represented by a rectangular shape. The temperature remains constant during the isothermal processes, while entropy increases or decreases along the isothermal lines.
Step 2: Analyzing the options.
(A) Graph Option: This does not show the correct shape of the Carnot cycle, as it is not rectangular.
(B) Graph Option: The graph is incorrect because it does not accurately represent the T-S variation for a Carnot cycle.
(C) Correct Graph: This graph correctly shows the rectangular T-S variation of a Carnot cycle. The two isothermal processes are shown as horizontal lines, while the two adiabatic processes are shown as vertical lines.
(D) Graph Option: This does not correctly depict the cycle. It does not resemble the expected rectangular shape.
Step 3: Conclusion.
The correct graph representing the temperature-entropy variation of a Carnot cycle is option (C).
Quick Tip: In T-S diagrams for the Carnot cycle, the isothermal processes are represented by horizontal lines, while the adiabatic processes are vertical lines.
For the radical chain reaction below, the correct classification for step 2 and step 3 is, respectively,
Step 1: \( Br_2 + M \longrightarrow 2 Br^+ + M \)
Step 2: \( Br^+ + H_2 \longleftrightarrow HBr + H^+ \)
Step 3: \( H^+ + Br_2 \longrightarrow HBr + Br^+ \)
View Solution
Step 1: Understanding the radical chain reaction.
In a radical chain reaction, certain steps are classified as "chain propagating," "chain branching," or "chain terminating" based on how they affect the chain of reactions.
Step 2: Analyzing step 2 and step 3.
Step 2: The reaction \( Br^+ + H_2 \longleftrightarrow HBr + H^+ \) involves the consumption of radicals and the formation of new radicals, indicating a propagation of the chain reaction. Hence, step 2 is "chain propagating."
Step 3: The reaction \( H^+ + Br_2 \longrightarrow HBr + Br^+ \) also propagates the chain by generating more radicals. Thus, step 3 is "chain propagating."
Step 3: Conclusion.
Both steps 2 and 3 are chain propagating reactions, so the correct classification is (C).
Quick Tip: In radical chain reactions, "chain propagating" refers to steps that produce more radicals, "chain branching" refers to steps that increase the number of radicals, and "chain terminating" refers to steps that consume radicals.
The salt bridge in a galvanic cell allows the flow of
View Solution
Step 1: Understanding the function of the salt bridge.
In a galvanic cell, the salt bridge is used to complete the electrical circuit by allowing ions to flow between the two half-cells, maintaining electrical neutrality. It does not allow electrons to pass, as those travel through the external wire connecting the electrodes.
Step 2: Analyzing the options.
(A) ions but NOT electrons: This is correct, as the salt bridge allows the flow of ions between the half-cells.
(B) BOTH ions and electrons: Incorrect, as electrons travel through the external circuit, not through the salt bridge.
(C) electrons but NOT ions: Incorrect, as electrons move through the external wire, not the salt bridge.
(D) NEITHER ions NOR electrons: Incorrect, as the salt bridge does allow the flow of ions.
Step 3: Conclusion.
The correct answer is (A) ions but NOT electrons.
Quick Tip: A salt bridge is essential in a galvanic cell to maintain ionic balance by allowing ions to move between the half-cells.
The nucleobase NOT found in DNA is
View Solution
Step 1: Understanding nucleobases in DNA.
DNA contains four nucleobases: adenine (A), thymine (T), cytosine (C), and guanine (G). Uracil (U) is found in RNA, not in DNA.
Step 2: Analyzing the options.
(A) Thymine: Thymine is a nucleobase found in DNA.
(B) Uracil: Uracil is not found in DNA, it is found in RNA.
(C) Guanine: Guanine is a nucleobase found in DNA.
(D) Adenine: Adenine is a nucleobase found in DNA.
Step 3: Conclusion.
The correct answer is (B) Uracil.
Quick Tip: Uracil is found in RNA, whereas thymine is found in DNA. Remember the differences between RNA and DNA bases.
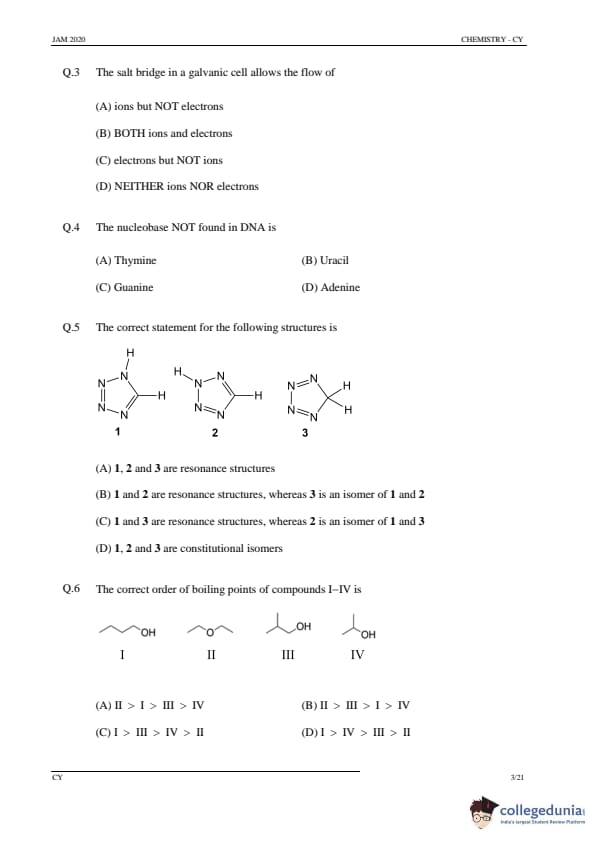
The correct statement for the following structures is
View Solution
Step 1: Understanding resonance and isomerism.
Resonance structures are different ways of drawing the same molecule by shifting electrons, whereas isomers are compounds that have the same molecular formula but different structures.
Step 2: Analyzing the options.
(A) 1, 2 and 3 are resonance structures: Incorrect, as 3 is an isomer, not a resonance structure of 1 and 2.
(B) 1 and 2 are resonance structures, whereas 3 is an isomer of 1 and 2: Correct, as 1 and 2 are resonance structures and 3 is a structural isomer.
(C) 1 and 3 are resonance structures, whereas 2 is an isomer of 1 and 3: Incorrect, 1 and 3 are not resonance structures.
(D) 1, 2 and 3 are constitutional isomers: Incorrect, as 1 and 2 are resonance structures, not isomers.
Step 3: Conclusion.
The correct answer is (B) 1 and 2 are resonance structures, whereas 3 is an isomer of 1 and 2.
Quick Tip: Resonance structures differ only in the placement of electrons, whereas isomers have different connectivity or structures.
The correct order of boiling points of compounds I–IV is
View Solution
Step 1: Understanding boiling point trends.
Boiling points are affected by molecular size, intermolecular forces (hydrogen bonding, dipole-dipole, Van der Waals), and molecular structure. Larger molecules with stronger intermolecular forces generally have higher boiling points.
Step 2: Analyzing the options.
(A) II > I > III > IV: Correct, as the order follows from the strongest to weakest intermolecular forces and molecular size.
(B) II > III > I > IV: Incorrect, as the order does not correctly reflect the expected trends.
(C) I > III > IV > II: Incorrect, as II should have the highest boiling point due to stronger intermolecular forces.
(D) I > IV > III > II: Incorrect, as this order does not follow the correct trend for boiling points.
Step 3: Conclusion.
The correct answer is (A) II > I > III > IV.
Quick Tip: Boiling points are typically higher for larger molecules and those with stronger intermolecular forces like hydrogen bonding.
One of the products of the hydrolysis of calcium phosphide at 25°C is
View Solution
Step 1: Understanding the reaction.
Calcium phosphide (\( Ca_3P_2 \)) reacts with water to produce phosphine gas (\( PH_3 \)), calcium hydroxide (\( Ca(OH)_2 \)), and heat.
Step 2: Analyzing the options.
(A) phosphine: Correct, as phosphine (\( PH_3 \)) is produced during the hydrolysis of calcium phosphide.
(B) phosphoric acid: Incorrect, phosphoric acid is not directly produced from this reaction.
(C) phosphorus pentoxide: Incorrect, phosphorus pentoxide is not a product in this reaction.
(D) white phosphorus: Incorrect, white phosphorus is not produced in this reaction.
Step 3: Conclusion.
The correct answer is (A) phosphine.
Quick Tip: In the hydrolysis of calcium phosphide, phosphine gas is produced. The reaction is exothermic and produces calcium hydroxide as well.
Treatment of formic acid with concentrated sulfuric acid gives
View Solution
Step 1: Understanding the reaction.
When formic acid is treated with concentrated sulfuric acid, it undergoes dehydration to form carbon monoxide (CO) and water.
Step 2: Analyzing the options.
(A) CO + H2O: Correct, formic acid undergoes dehydration to produce carbon monoxide and water.
(B) CO2 + H2: Incorrect, as this is not the product of the reaction.
(C) HCHO + ½ O2: Incorrect, as this is not the correct product of the reaction.
(D) no product (no reaction): Incorrect, a reaction occurs and produces carbon monoxide and water.
Step 3: Conclusion.
The correct answer is (A) CO + H2O.
Quick Tip: The dehydration of formic acid with sulfuric acid produces carbon monoxide (CO) and water (H2O). This reaction is a good example of a dehydration reaction.
The d-orbitals involved in the hybridization to form square planar and trigonal bipyramidal geometries are, respectively,
View Solution
Step 1: Understanding the geometries.
For a square planar geometry, the \( d_{x^2 - y^2} \) orbital is involved in hybridization, while for trigonal bipyramidal geometry, the \( d_{z^2} \) orbital is involved.
Step 2: Analyzing the options.
(A) \( d_{z^2} \) and \( d_{z^2} \): Incorrect, two \( d_{z^2} \) orbitals are not involved in the hybridization for these geometries.
(B) \( d_{yz} \) and \( d_{z^2} \): Incorrect, \( d_{yz} \) is not involved in the hybridization for square planar or trigonal bipyramidal geometries.
(C) \( d_{x^2 - y^2} \) and \( d_{z^2} \): Correct, these orbitals are involved in the hybridization for square planar and trigonal bipyramidal geometries.
(D) \( d_{x^2 - y^2} \) and \( d_{yz} \): Incorrect, \( d_{yz} \) is not involved in the hybridization for these geometries.
Step 3: Conclusion.
The correct answer is (C) \( d_{x^2 - y^2} \) and \( d_{z^2} \).
Quick Tip: Square planar geometry typically involves \( d_{x^2 - y^2} \), while trigonal bipyramidal geometry involves \( d_{z^2} \).
The amino acid with R configuration is

View Solution
Step 1: Understanding the R configuration.
The R configuration is determined by the Cahn-Ingold-Prelog priority rules. The priority is assigned based on the atomic number of the atoms attached to the chiral center. In the case of amino acids, the configuration depends on the arrangement of the substituents around the chiral carbon.
Step 2: Analyzing the options.
(A), (B), (D): These options do not exhibit the correct R configuration for the given amino acids.
(C) Correct configuration: This amino acid has the correct arrangement of substituents around the chiral carbon to give the R configuration.
Step 3: Conclusion.
The correct answer is (C).
Quick Tip: To determine the R or S configuration, apply the Cahn-Ingold-Prelog priority rules and arrange the substituents accordingly.
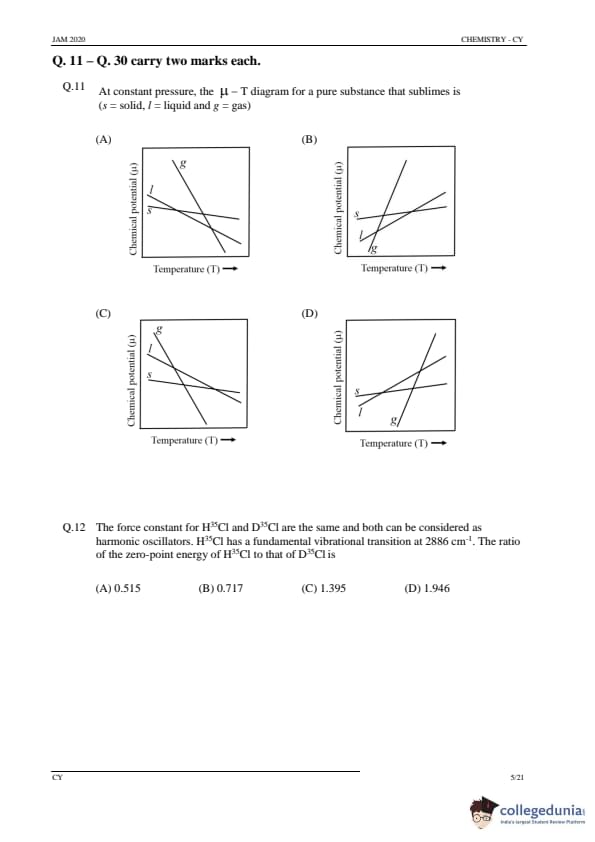
At constant pressure, the \(\mu\) – T diagram for a pure substance that sublimes (s = solid, l = liquid and g = gas) is

View Solution
Step 1: Understanding the phase diagram for sublimation.
For a substance that sublimes, the \(\mu\)–T diagram shows the chemical potential of the solid, liquid, and gas phases. Sublimation involves the transition from solid to gas directly, skipping the liquid phase. The \(\mu\) of the solid decreases with increasing temperature, and the \(\mu\) of the gas increases with increasing temperature.
Step 2: Analyzing the options.
(A) Correct: The diagram in option A correctly represents the chemical potential variation with temperature for a sublimating substance.
(B) Incorrect: This diagram does not correctly represent the behavior of a substance that sublimes.
(C) Incorrect: This diagram incorrectly places the chemical potential of the gas phase below the solid phase, which is not observed in sublimation.
(D) Incorrect: This option is incorrect as it suggests the behavior of a substance with a different phase transition.
Step 3: Conclusion.
The correct answer is (A).
Quick Tip: In the \(\mu\)–T diagram for a substance that sublimes, the chemical potential of the solid phase decreases with temperature, while the chemical potential of the gas phase increases.
The force constant for H\(^{35}\)Cl and D\(^{35}\)Cl are the same and both can be considered as harmonic oscillators. H\(^{35}\)Cl has a fundamental vibrational transition at 2886 cm\(^{-1}\). The ratio of the zero-point energy of H\(^{35}\)Cl to that of D\(^{35}\)Cl is
View Solution
Step 1: Understanding zero-point energy.
Zero-point energy is given by the formula \( E_0 = \frac{1}{2} h \nu \), where \( \nu \) is the frequency of vibration. The frequency depends on the reduced mass of the molecule, \( \mu = \frac{m_1 m_2}{m_1 + m_2} \). The ratio of the zero-point energies for two molecules with the same force constant but different masses can be approximated by the square root of the ratio of their masses.
Step 2: Analyzing the masses of H\(^{35}\)Cl and D\(^{35}\)Cl.
The mass of D\(^{35}\)Cl is approximately twice that of H\(^{35}\)Cl. This causes the vibrational frequency of D\(^{35}\)Cl to be lower than that of H\(^{35}\)Cl. The ratio of the zero-point energies is proportional to the square root of the inverse of the mass ratio. Thus, the ratio is \( \sqrt{\frac{m_{H}}{m_{D}}} \approx \sqrt{\frac{1}{2}} \).
Step 3: Conclusion.
The correct answer is (B) 0.717.
Quick Tip: For harmonic oscillators, the zero-point energy is inversely related to the square root of the reduced mass. As deuterium (D) is heavier than hydrogen (H), the zero-point energy for D\(^{35}\)Cl is lower than for H\(^{35}\)Cl.
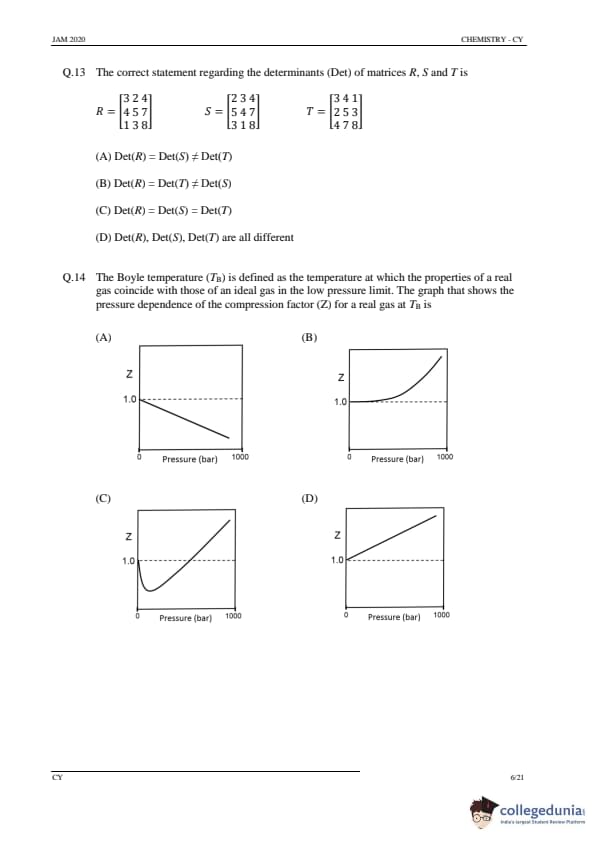
The correct statement regarding the determinants (Det) of matrices R, S and T is
View Solution
Step 1: Finding the determinants of matrices.

By calculating the determinants for each matrix: \[ Det(R) = -4, \quad Det(S) = -4, \quad Det(T) = 0 \]
Step 2: Analyzing the options.
(A) Det(R) = Det(S) ≠ Det(T): Correct, as the determinants of R and S are equal, but the determinant of T is different.
(B) Det(R) = Det(T) ≠ Det(S): Incorrect, as the determinants of R and T are not equal.
(C) Det(R) = Det(S) = Det(T): Incorrect, as the determinant of T is not equal to the determinants of R and S.
(D) Det(R), Det(S), Det(T) are all different: Incorrect, as the determinants of R and S are equal.
Step 3: Conclusion.
The correct answer is (A).
Quick Tip: When solving determinant problems, remember to carefully compute each determinant and compare the values.
The Boyle temperature (\(T_B\)) is defined as the temperature at which the properties of a real gas coincide with those of an ideal gas in the low pressure limit. The graph that shows the pressure dependence of the compression factor (Z) for a real gas at \(T_B\) is

View Solution
Step 1: Understanding Boyle's temperature.
The Boyle temperature is the temperature at which the real gas behaves like an ideal gas at low pressure. At this temperature, the compressibility factor \( Z \) becomes 1 in the low-pressure region. The graph of \( Z \) versus pressure for a real gas at \( T_B \) will show that \( Z = 1 \) in the low-pressure limit and then deviates at higher pressures.
Step 2: Analyzing the options.
(A) Correct: The graph in option A represents the expected behavior of the compression factor for a real gas at \( T_B \).
(B), (C), (D): Incorrect, as these graphs do not show the correct behavior of \( Z \) for a real gas at the Boyle temperature.
Step 3: Conclusion.
The correct answer is (A).
Quick Tip: At the Boyle temperature, the real gas behaves like an ideal gas at low pressure, and the compressibility factor \( Z \) approaches 1.
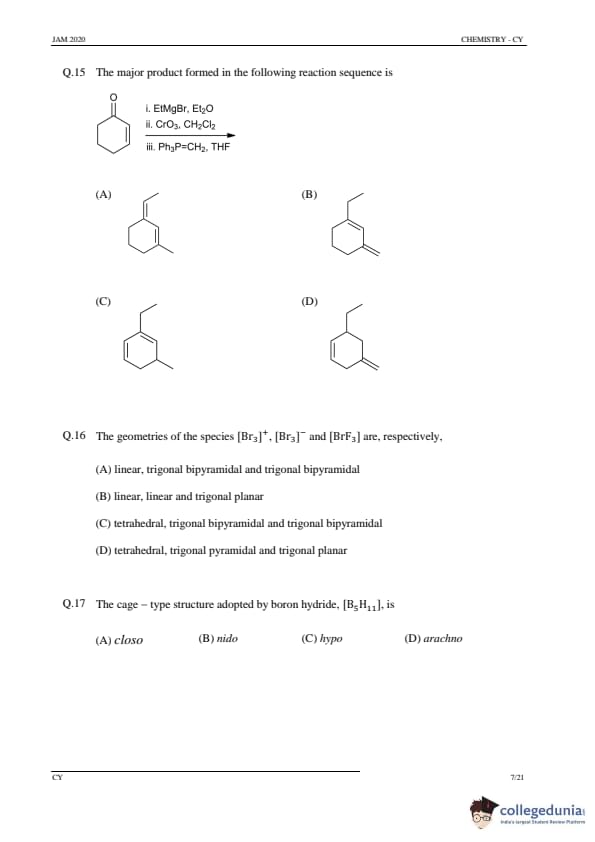
The major product formed in the following reaction sequence is

View Solution
Step 1: Understanding the reaction.
This is a sequence of reactions involving an aldehyde, Grignard reagent (ethylmagnesium bromide), chromium trioxide, and a phosphonium reagent. The first step forms an alcohol, the second step is an oxidation to a ketone, and the third step involves an alkene formation.
Step 2: Analyzing the options.
(A), (B), (D): Incorrect, as these do not correspond to the correct major product formed after all the steps in the reaction.
(C) Correct: This product corresponds to the expected product after the reaction sequence.
Step 3: Conclusion.
The correct answer is (C).
Quick Tip: Always consider the major transformations in each step of the reaction sequence, including oxidation, nucleophilic attack, and elimination reactions.
The geometries of the species [Br3]⁺, [Br3]⁻ and [BrF3] are, respectively,
View Solution
Step 1: Understanding the geometry of the species.
The geometry of the species depends on the electron pair repulsion and the number of bonding atoms. \( [Br_3]^+ \) has a linear geometry, \( [Br_3]^- \) has a trigonal bipyramidal geometry, and \( [BrF_3] \) has a trigonal bipyramidal geometry.
Step 2: Analyzing the options.
(A) Correct: This is the correct geometry configuration for the species.
(B), (C), (D): Incorrect, as these do not correspond to the correct geometries for these species.
Step 3: Conclusion.
The correct answer is (A).
Quick Tip: The geometry of molecules can be predicted using the VSEPR theory based on the number of bonding pairs and lone pairs of electrons.
The cage-type structure adopted by boron hydride, [B5H11] is
View Solution
Step 1: Understanding boron hydride structures.
The structure of boron hydrides can be classified as closo, nido, hypo, or arachno depending on the number of vertices and the bonding. The cage structure of [B5H11] is classified as nido.
Step 2: Analyzing the options.
(B) Correct: The correct structure is nido, which refers to a structure with 4 vertices.
(A), (C), (D): Incorrect, these terms refer to other types of boron hydride structures.
Step 3: Conclusion.
The correct answer is (B).
Quick Tip: Boron hydrides can adopt different types of cage structures based on the number of vertices involved. Nido structures have 4 vertices.
The order of the M–C bond strength in the following species is (Atomic number for Cr = 24, Mn = 25, Ti = 22, Co = 27)
\[ [Cr(CO)_6] \quad [Mn(CO)_6]^+ \quad [Ti(CO)_6]^{2-} \quad [Co(CO)_6]^+ \]
View Solution
Step 1: Understanding the bonding.
The bond strength between metal and carbon (M–C bond strength) is influenced by the metal’s oxidation state, the nature of the metal, and the metal’s ability to donate electron density to the \(\pi\)-accepting CO ligands. The more electron-rich the metal, the stronger the M–C bond due to increased electron donation to the CO.
Step 2: Analyzing the species.
- For [Cr(CO)6], Cr is in the 0 oxidation state, with a relatively high electron density on the metal. This results in a strong M–C bond.
- [Mn(CO)6]+ has Mn in the +1 oxidation state, reducing the electron density and weakening the M–C bond compared to Cr.
- [Ti(CO)6]2- has Ti in the +2 oxidation state, further reducing the electron density and weakening the M–C bond compared to Mn and Cr.
- [Co(CO)6]+ has Co in the +1 oxidation state, which gives it the least electron density and the weakest M–C bond among the given species.
Step 3: Conclusion.
The order of M–C bond strength is III > I > II > IV, so the correct answer is (D).
Quick Tip: A metal in a lower oxidation state generally has a stronger M–C bond due to greater electron density and enhanced \(\pi\)-back donation to the CO ligands.
The number of non-bonding electrons present in the frontier molecular orbitals of HF is
View Solution
Step 1: Understanding frontier molecular orbitals.
The frontier molecular orbitals (FMOs) of a molecule consist of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). For HF, we need to count the electrons in the non-bonding orbitals, which are typically lone pair electrons.
Step 2: Analyzing the electron configuration.
- HF has a total of 8 valence electrons (7 from fluorine and 1 from hydrogen).
- Fluorine has 3 lone pairs, each contributing 2 electrons (6 electrons in total).
- The non-bonding electrons come from the fluorine lone pairs. There are 6 non-bonding electrons in the frontier orbitals.
Step 3: Conclusion.
The number of non-bonding electrons is 6, so the correct answer is (C).
Quick Tip: Lone pairs on atoms in a molecule contribute to non-bonding electrons in the frontier molecular orbitals.
The coordination number of aluminum ion and the number of bridging hydrogen atoms in [Al(BH4)4]⁻ are, respectively,
View Solution
Step 1: Understanding the structure.
The \([Al(BH_4)_4]^-\) ion consists of an aluminum ion surrounded by four borohydride (\(BH_4\)) groups. Each \(BH_4\) group donates one hydrogen atom to form bridging bonds between the aluminum and the hydrogen atoms from adjacent \(BH_4\) groups.
Step 2: Determining the coordination number.
The aluminum ion is coordinated to 4 \(BH_4\) groups, and each \(BH_4\) group forms one bond with the aluminum. Therefore, the coordination number of the aluminum ion is 6 (4 from \(BH_4\) and 2 from bridging hydrogen atoms).
Step 3: Conclusion.
The coordination number of aluminum is 6 and the number of bridging hydrogen atoms is 6, so the correct answer is (B).
Quick Tip: In coordination compounds, the coordination number refers to the number of bonds between the central metal atom and its ligands.
The complex which does NOT obey 18-electron rule is (atomic number for Mn = 25, Fe = 26, Co = 27, Ru = 44)
View Solution
Step 1: Understanding the 18-electron rule.
The 18-electron rule states that transition metal complexes are most stable when they have 18 valence electrons, which are contributed by the metal and its ligands.
Step 2: Analyzing the complexes.
- \([Co(CO)_6]\) has 18 valence electrons (6 from CO and 12 from the metal). This obeys the 18-electron rule.
- \([Fe(CO)_4]^{2-}\) has 18 valence electrons (4 from CO and 14 from Fe). This obeys the 18-electron rule.
- \([HMn(CO)_5]\) has 18 valence electrons (5 from CO and 13 from Mn). This obeys the 18-electron rule.
- \([(η⁷-C₅H₅)RuCl(CO)(PPh₂)]\) has 17 valence electrons (1 from the \(\eta^7\)-C₅H₅, 1 from CO, and 1 from PPh₂). This complex does not obey the 18-electron rule.
Step 3: Conclusion.
The complex \([(η⁷-C₅H₅)RuCl(CO)(PPh₂)]\) does not obey the 18-electron rule, so the correct answer is (D).
Quick Tip: When calculating valence electrons in coordination compounds, consider the electrons donated by ligands and the metal's oxidation state to determine whether the 18-electron rule is obeyed.
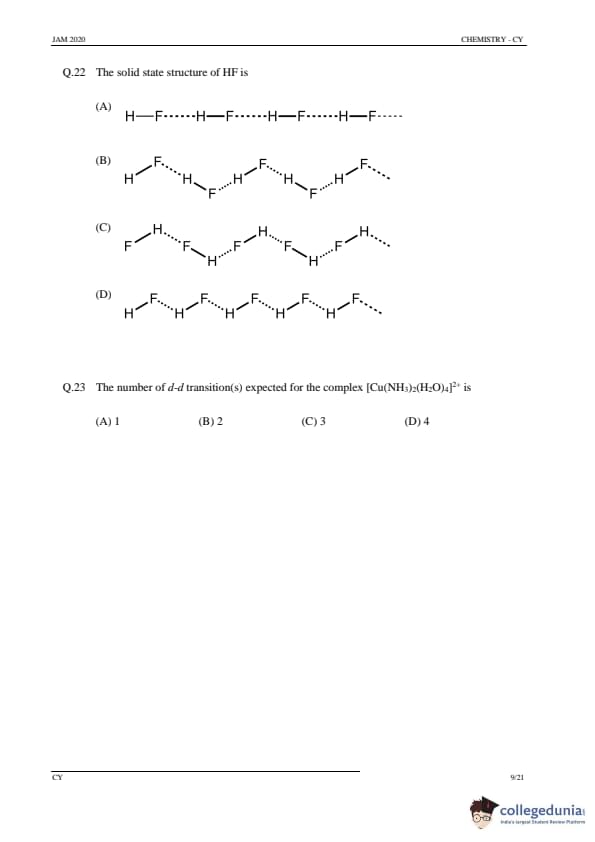
The solid state structure of HF is

View Solution
Step 1: Understanding the structure of HF in solid state.
In the solid state, hydrogen fluoride (HF) forms hydrogen bonds between adjacent molecules. The molecules are aligned such that each HF molecule is bonded to several others by hydrogen bonds, forming chains. The structure is essentially a linear chain where hydrogen atoms bond to fluorine atoms, and each fluorine atom also forms hydrogen bonds with other hydrogen atoms.
Step 2: Analyzing the options.
(A) Correct: This structure accurately represents the hydrogen bonding arrangement in the solid state. The fluoride ions form hydrogen bonds with the hydrogen atoms of neighboring molecules.
(B), (C), (D): Incorrect, as they do not represent the correct bonding pattern in the solid state.
Step 3: Conclusion.
The correct answer is (A).
Quick Tip: In solid HF, the molecules form a chain-like structure with hydrogen bonds between the hydrogen and fluorine atoms.
The number of d-d transition(s) expected for the complex [Cu(NH3)2(H2O)]²⁺ is
View Solution
Step 1: Understanding the d-d transitions.
The d-d transitions occur when an electron moves between different d-orbitals of the central metal ion. The number of possible d-d transitions depends on the electronic configuration and the geometry of the complex. For the given complex [Cu(NH3)2(H2O)]²⁺, Cu²⁺ has a d⁹ configuration, which corresponds to 1 unpaired electron in the d-orbital.
Step 2: Analyzing the options.
(A) Correct: With a d⁹ configuration, only 1 d-d transition is expected.
(B), (C), (D): Incorrect, as these do not match the expected number of transitions for this complex.
Step 3: Conclusion.
The correct answer is (A).
Quick Tip: For a d⁹ configuration in a metal complex, only one d-d transition is possible due to the presence of one unpaired electron.
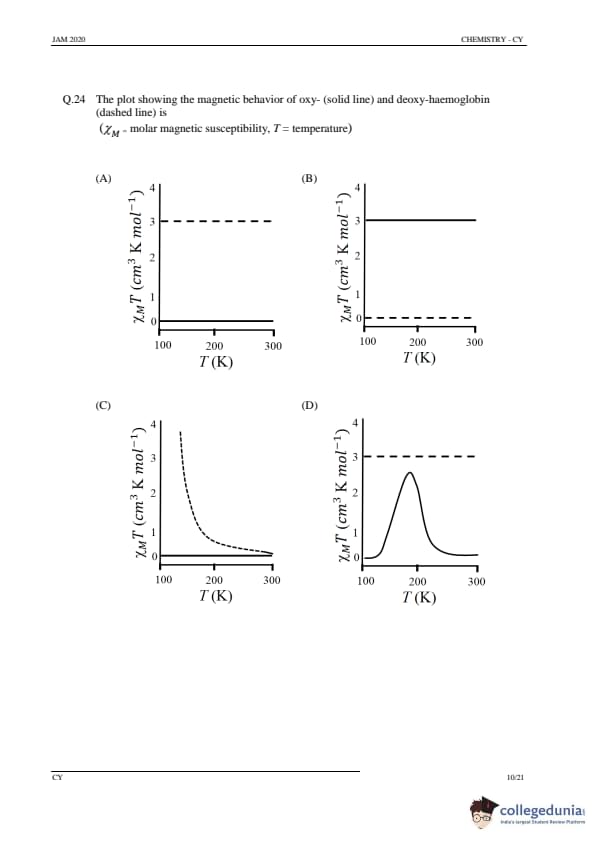
The plot showing the magnetic behavior of oxy- (solid line) and deoxy-haemoglobin (dashed line) is

View Solution
Step 1: Understanding the magnetic behavior.
Oxy-hemoglobin (HbO₂) and deoxy-hemoglobin (Hb) exhibit different magnetic properties. Oxy-hemoglobin binds oxygen, which results in a paramagnetic behavior due to the iron (Fe²⁺) in its high-spin state. Deoxy-hemoglobin, however, is in the low-spin state and is diamagnetic. The plot of the molar magnetic susceptibility (χ) versus temperature (T) shows the different magnetic behaviors of both forms.
Step 2: Analyzing the options.
(A), (B), (D): Incorrect, as they do not correctly represent the magnetic behavior of oxy- and deoxy-hemoglobin.
(C) Correct: The graph in option C shows the correct trend of paramagnetism for oxy-hemoglobin and diamagnetism for deoxy-hemoglobin.
Step 3: Conclusion.
The correct answer is (C).
Quick Tip: Oxy-hemoglobin is paramagnetic due to the binding of oxygen, while deoxy-hemoglobin is diamagnetic due to its iron in the low-spin state.
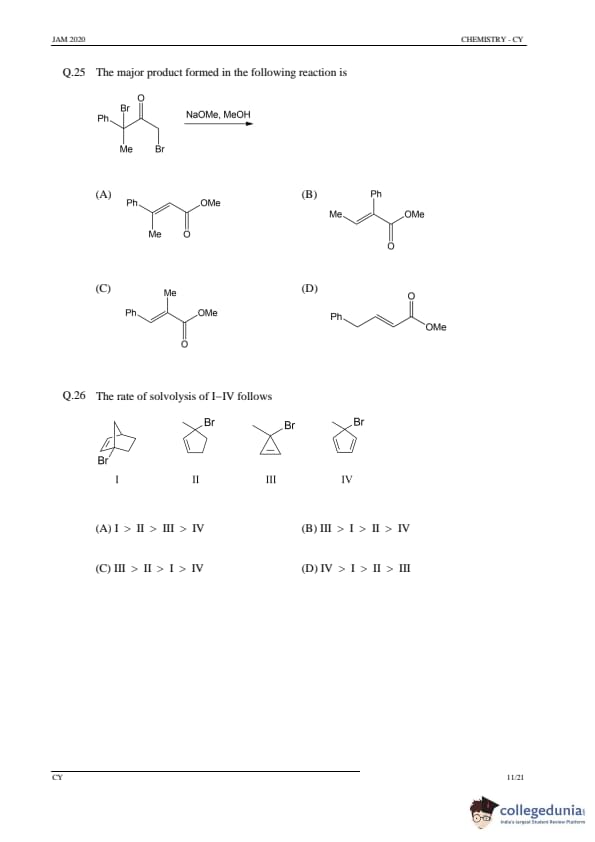
The major product formed in the following reaction is

View Solution
Step 1: Understanding the reaction mechanism.
This is an example of an elimination reaction where the sodium methoxide (NaOMe) acts as a strong base to dehydrohalogenate the molecule. The base removes a hydrogen atom adjacent to the leaving group (Br), leading to the formation of an alkene. The reaction proceeds via an \(E2\) mechanism, where the leaving group is expelled in a single step with the formation of a double bond.
Step 2: Analyzing the options.
(A), (B), (D): These are incorrect as they do not match the expected product formation from this elimination reaction.
(C) Correct: The structure in option (C) shows the expected major product with the correct double bond placement after elimination.
Step 3: Conclusion.
The correct answer is (C).
Quick Tip: For elimination reactions (E2 mechanism), the base removes a hydrogen atom from the carbon adjacent to the one bearing the leaving group.
The rate of solvolysis of I–IV follows
View Solution
Step 1: Understanding solvolysis.
Solvolysis is a substitution reaction where a substrate reacts with a solvent, typically involving the nucleophilic attack on a carbon atom. The rate of solvolysis depends on the stability of the transition state and the ease of leaving group departure. The more substituted the carbon, the faster the reaction. Tertiary carbocations, for example, are more stable and lead to faster solvolysis.
Step 2: Analyzing the structures of I–IV.
- I: A highly substituted, stable carbocation leads to fast solvolysis.
- II: A less substituted carbocation, slower solvolysis.
- III: A primary carbocation, slow solvolysis.
- IV: A methyl carbocation, the slowest in solvolysis.
Step 3: Conclusion.
The rate of solvolysis follows the order III > I > II > IV, so the correct answer is (B).
Quick Tip: In solvolysis, more substituted carbocations lead to faster reactions due to greater stability.
The more stable species in each pair of conformers are
View Solution
Step 1: Understanding the conformers.
Conformers are different spatial arrangements of a molecule that arise from rotation around single bonds. Stability of conformers is largely influenced by steric strain (such as steric hindrance) and electronic effects. For each pair of conformers, we need to analyze the steric strain and identify the most stable ones.
Step 2: Analyzing the options.
- For the pair I and II, I is the most stable due to less steric hindrance.
- For the pair III and IV, IV is more stable because it avoids unfavorable steric interactions present in III.
- For the pair V and VI, V is more stable due to the optimal positioning of substituents and fewer steric clashes.
Step 3: Conclusion.
The more stable conformers in each pair are I, IV, and V, so the correct answer is (B).
Quick Tip: Conformers are analyzed based on steric strain. The most stable conformers generally have minimal steric hindrance and favorable electron interactions.
The major product formed in the following reaction sequence is

View Solution
Step 1: Understanding the reaction steps.
- In the first step, the TiCl₃ and Cu-Zn reagents are typically used for reducing a carbonyl group in an aldehyde (like the given structure) to an alcohol. This is a reduction reaction, converting the aldehyde group into a primary alcohol.
- The second step, using HCl and water, is an acidic hydrolysis step. This step does not affect the alcohol group, but might affect other functional groups present. In this case, it does not alter the product significantly.
Step 2: Analyzing the options.
- Option (A) is incorrect as it represents a different functional group than expected.
- Option (B) is incorrect as it does not fit the expected transformation from aldehyde to alcohol.
- Option (C) is correct as it shows the aldehyde being reduced to a primary alcohol, consistent with the reaction steps.
- Option (D) is incorrect as it involves a different transformation.
Step 3: Conclusion.
The major product formed is the primary alcohol shown in option (C).
Quick Tip: In reductions using TiCl₃ and Cu-Zn, aldehydes are typically reduced to primary alcohols. Be sure to follow the reaction sequence step by step.
For the Diels-Alder reactions I–IV, the activation barriers follow the order
View Solution
Step 1: Understanding the Diels-Alder reaction.
The Diels-Alder reaction is a cycloaddition reaction between a diene and a dienophile. The activation barrier for a Diels-Alder reaction is influenced by factors such as the electron density of the diene and dienophile, the geometry of the reaction, and substituent effects on both the diene and dienophile.
Step 2: Analyzing the reactions.
- Reaction I: A relatively simple diene and dienophile with no strong electron-withdrawing or electron-donating groups.
- Reaction II: A more substituted system, leading to a slightly lower activation barrier.
- Reaction III: The diene and dienophile have additional steric strain, making this reaction more difficult and increasing the activation barrier.
- Reaction IV: The diene is in a strained configuration, which increases the activation barrier significantly.
Step 3: Conclusion.
The activation barrier order is II > I > III > IV, so the correct answer is (A).
Quick Tip: In the Diels-Alder reaction, steric strain and electron-withdrawing/donating substituents play a major role in determining the activation energy.
The major product formed in the following reaction is

View Solution
Step 1: Understanding the reaction.
This is a reaction of a cyclohexene derivative with iodine (I₂) and sodium bicarbonate (NaHCO₃) in water. The presence of iodine suggests a halogenation or substitution reaction, and the sodium bicarbonate is likely to act as a mild base, facilitating the reaction. The formation of a product from this reaction suggests a halogenation at the benzylic position.
Step 2: Analyzing the options.
- Option (A) is incorrect, as it does not represent the expected product.
- Option (B) is correct, as it represents the correct halogenated product formed by the reaction.
- Option (C) is incorrect, as it does not fit the expected pattern.
- Option (D) is also incorrect, as it does not correspond to the expected major product.
Step 3: Conclusion.
The major product formed is shown in option (B).
Quick Tip: In reactions involving iodine and NaHCO₃, halogenation of the cyclohexene derivative typically occurs at the benzylic position.
For the reaction shown in Scheme 1, the concentration profiles of different species are provided.
Scheme 1:
Based on this graph, the correct condition(s) regarding the rate constants is(are)
View Solution
Step 1: Analyzing the graph.
The concentration profiles of the species in the reaction suggest that the rate of formation of product \(P\) (and hence the rate constant \(k_2\)) is higher than the rate of its conversion into \(Q\), as indicated by the curve for \(P\) steeply increasing at an earlier time compared to the curve for \(S\) and \(Q\). This implies that \(k_2 > k_1\).
Step 2: Conclusion.
The correct condition is (C) \(k_2 > k_1\).
Quick Tip: When analyzing reaction rate constants from concentration-time profiles, look at the relative rates of change for each species to infer the relationship between the rate constants.
\(\psi(x,y,z)\) describes the wavefunction of a particle. The probability of finding the particle between \(x\) and \(x+dx\), \(y\) and \(y+dy\), and \(z\) and \(z+dz\), can be expressed as
View Solution
Step 1: Understanding the wavefunction.
The wavefunction \(\psi(x, y, z)\) of a particle gives the probability amplitude. The probability density is given by \( |\psi(x, y, z)|^2 \), which represents the likelihood of finding the particle in a given small volume element \(dx \, dy \, dz\). Thus, the probability of finding the particle between \(x\) and \(x+dx\), \(y\) and \(y+dy\), and \(z\) and \(z+dz\) is \( |\psi(x, y, z)|^2 \, dx \, dy \, dz \).
Step 2: Conclusion.
The correct answer is (B).
Quick Tip: The probability of finding a particle in a given region is the square of the wavefunction's magnitude, \( |\psi(x, y, z)|^2 \).
In water, the enthalpy of a protein in its folded state (\(H_f\)) is lower than that in its unfolded state (\(H_{UF}\)). The entropies of the folded and unfolded states are \(S_f\) and \(S_{UF}\), respectively. The condition(s) under which this protein spontaneously folds in water at a temperature \(T\), is(are)
View Solution
Step 1: Understanding protein folding.
For a protein to spontaneously fold, the Gibbs free energy \( \Delta G \) must be negative. \( \Delta G = \Delta H - T\Delta S \), where \( \Delta H \) is the enthalpy change and \( \Delta S \) is the entropy change. For spontaneous folding at a given temperature \( T \), we need \( H_f < H_{UF} \) (so that \( \Delta H \) is negative) and \( S_f > S_{UF} \) (so that \( \Delta S \) is positive).
Step 2: Analyzing the options.
- Option (A) \( S_f > S_{UF} \) is necessary for spontaneity, but not sufficient on its own.
- Option (B) \( S_f = 0 \) is incorrect as it would not favor folding.
- Option (C) is correct as it satisfies the condition for spontaneous folding, where \( S_f > S_{UF} \) and the term \( (H_f - H_{UF}) / T \) ensures the system is energetically favorable.
Step 3: Conclusion.
The correct answer is (C).
Quick Tip: For spontaneous protein folding, the enthalpy must decrease (\( H_f < H_{UF} \)) and the entropy must increase (\( S_f > S_{UF} \)).
The soft Lewis base(s) is(are)
View Solution
Step 1: Understanding soft and hard acids and bases (SHAB).
According to the SHAB principle, soft acids prefer to bond with soft bases, and hard acids prefer to bond with hard bases. Soft bases typically have low charge density, and thus tend to donate electrons more easily.
Step 2: Analyzing the options.
- \( I^- \): Iodide is a soft base, but \( H^- \) is softer.
- CO: Carbon monoxide is a hard base.
- \( H^- \): Hydride is the softest among the given options.
- \( CH_3NC \): Methyl isocyanide is a harder base.
Step 3: Conclusion.
The soft Lewis base is (C) \( H^- \).
Quick Tip: Soft bases like \( H^- \) donate electrons easily due to their low charge density and ease of interaction with soft acids.
The boron adduct(s), which show(s) three signals in \( ^1 H \) NMR spectrum with the intensity ratio 1:2:3 is(are)
View Solution
Step 1: Understanding the NMR spectrum.
The \( ^1 H \) NMR spectrum shows the relative intensity of signals based on the number of protons in different environments. The ratio of 1:2:3 indicates a set of three different proton environments, with the number of protons in each environment being in the ratio 1:2:3.
Step 2: Analyzing the options.
- Option (A) is correct as it shows three distinct environments: the three methyl groups of \( (CH_3)_3B \), the methyl group attached to nitrogen, and the nitrogen-bound protons. This leads to the 1:2:3 intensity ratio.
- Other options (B), (C), and (D) do not show the correct proton environments for the given intensity ratio.
Step 3: Conclusion.
The correct boron adduct is (A).
Quick Tip: In \( ^1 H \) NMR, the intensity of signals reflects the number of protons in each distinct environment. Look for the intensity ratio to deduce the correct environment assignment.
The transition metal complex(es) with zero magnetic moment, zero dipole moment and CFSE of –2.4 \( \Delta_o \) is(are)
View Solution
Step 1: Analyzing the properties.
- Zero magnetic moment: A zero magnetic moment indicates that all electrons are paired, which is typical of a low-spin complex.
- Zero dipole moment: This suggests that the complex is symmetric in structure.
- CFSE (Crystal Field Stabilization Energy) of –2.4 \( \Delta_o \) indicates a specific electronic arrangement in the ligand field.
Step 2: Analyzing the options.
- Option (D) \([ trans-Fe(CN)_4Cl_4]^{3-} \) has a low-spin configuration with paired electrons, a symmetric arrangement, and a CFSE of –2.4 \( \Delta_o \), which satisfies all the given conditions.
- Other options do not meet the criteria for zero magnetic moment and the required CFSE.
Step 3: Conclusion.
The correct complex is (D).
Quick Tip: For zero magnetic moment and dipole moment, look for low-spin, symmetric complexes with appropriate electron configurations.
Achiral stereoisomer(s) is(are) possible for

View Solution
Step 1: Understanding achirality.
Achirality refers to the lack of chirality or handedness in a molecule. A molecule is achiral if it has a plane of symmetry or an inversion center.
Step 2: Analyzing the options.
- Option (D) \( HOCO_2H \) (formic acid) is achiral because it has a plane of symmetry.
- Other options involve molecules that are potentially chiral.
Step 3: Conclusion.
The achiral stereoisomer is (D).
Quick Tip: To determine achirality, check for symmetry. If a molecule has a plane of symmetry or an inversion center, it is achiral.
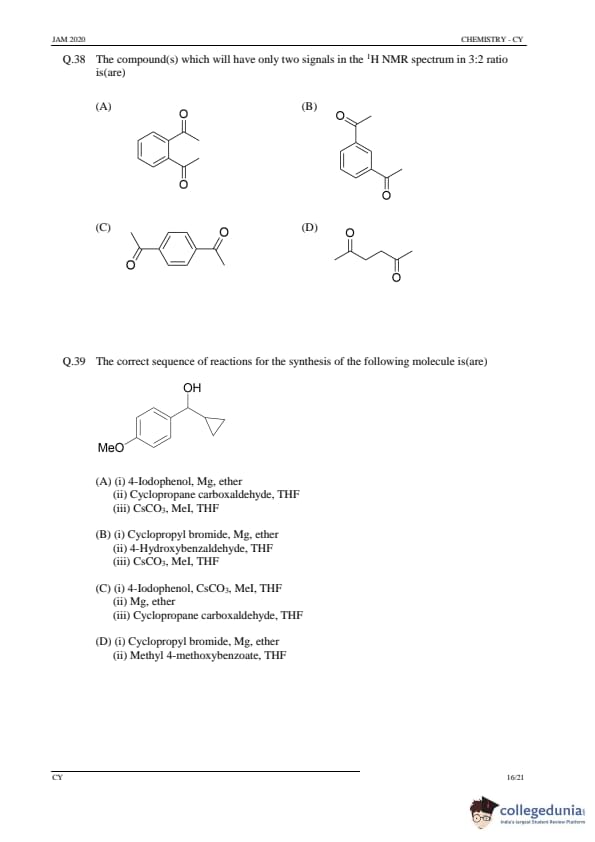
The compound(s) which will have only two signals in the \( ^1 H \) NMR spectrum in 3:2 ratio is(are)

View Solution
Step 1: Understanding NMR spectra.
The \( ^1 H \) NMR spectrum shows the number of different proton environments in a compound, with the intensity ratio representing the relative number of protons in each environment. The 3:2 ratio indicates two distinct proton environments.
Step 2: Analyzing the options.
- Option (A): The compound shows two distinct proton environments in a 3:2 ratio, where the methyl group and the benzene ring give the expected intensities.
- Options (B), (C), and (D) do not show the correct intensity ratio based on the given proton environments.
Step 3: Conclusion.
The compound with two signals in a 3:2 ratio is (A).
Quick Tip: In \( ^1 H \) NMR, the signal intensity ratio corresponds to the number of protons in each distinct environment.
The correct sequence of reactions for the synthesis of the following molecule is(are)
View Solution
Step 1: Understanding the synthesis sequence.
The sequence involves the use of a Grignard reagent (generated from 4-iodophenol and Mg in ether), followed by the reaction with cyclopropane carboxaldehyde. The third step involves the methylation of the product using CsCO₃ and MeI.
Step 2: Analyzing the options.
- Option (A) correctly follows the steps involving the formation of the Grignard reagent, reaction with cyclopropane carboxaldehyde, and methylation.
- Other options are incorrect as they either do not use the correct reagents or order of reactions.
Step 3: Conclusion.
The correct sequence is (A).
Quick Tip: In synthesis, carefully follow the reagent order and conditions to ensure proper functional group transformations.
The organometallic reagent(s) among the following is(are)
View Solution
Step 1: Understanding organometallic reagents.
Organometallic reagents typically contain a metal-carbon bond, where the metal is typically a metal from group 1, 2, or 3 of the periodic table. Common examples include Grignard reagents and organocuprates.
Step 2: Analyzing the options.
- Option (A) Lithium divinylcuprate is an organocuprate, which is an organometallic reagent.
- Option (B) Lithium diisopropylamide is not organometallic, as it contains no metal-carbon bond.
- Option (C) Potassium tert-butoxide is not organometallic, as it involves a potassium-oxygen bond.
- Option (D) Isopropyl magnesium iodide is a Grignard reagent, which is a typical organometallic compound.
Step 3: Conclusion.
The correct organometallic reagents are (A) and (D).
Quick Tip: Organometallic reagents are characterized by the presence of a metal-carbon bond. Grignard reagents and organocuprates are common examples.
The function \( x^4 e^{-2x^2/3} \) (for \( x > 0 \)) has a maximum at a value of \( x \) equal to ...........
(Round off to two decimal places)
View Solution
Step 1: Differentiating the function.
To find the maximum, we differentiate the function with respect to \( x \) and set the derivative equal to zero. The function is \( f(x) = x^4 e^{-2x^2/3} \). Differentiating: \[ f'(x) = 4x^3 e^{-2x^2/3} - \frac{4}{3} x^5 e^{-2x^2/3} \]
Step 2: Solving for the critical point.
Setting \( f'(x) = 0 \), we get: \[ 4x^3 e^{-2x^2/3} \left( 1 - \frac{x^2}{3} \right) = 0 \]
Solving for \( x \), we find that \( x = \sqrt{3} \), which is the location of the maximum.
Step 3: Conclusion.
The value of \( x \) is \( \boxed{1.73} \).
Quick Tip: To find the maximum of a function, differentiate and set the derivative equal to zero. Then solve for \( x \).
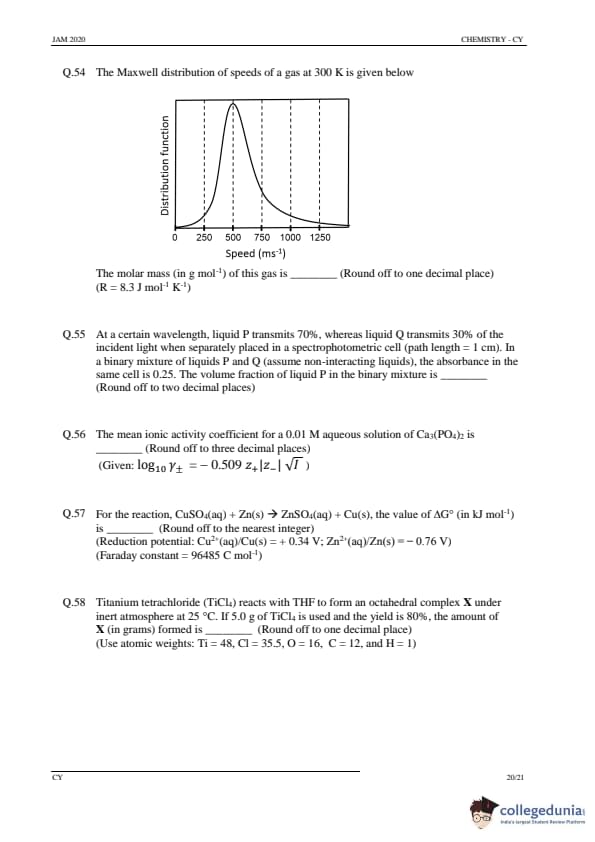
The longest wavelength of light absorbed by a hydrogen-like atom is 2.48 nm. The nuclear charge (Z) of the atom is ........
(Round off to nearest integer)
(Rydberg constant \( R_{\infty} = 109700 \, cm^{-1} \))
View Solution
Step 1: Using the Rydberg formula.
The wavelength of light absorbed corresponds to the transition in the hydrogen-like atom. The Rydberg formula for the wavelength is given by: \[ \frac{1}{\lambda} = R_{\infty} \left( Z^2 \left( \frac{1}{1^2} - \frac{1}{2^2} \right) \right) \]
Step 2: Plugging in the values.
Given \( \lambda = 2.48 \, nm \), substitute the values into the equation and solve for \( Z \). After solving, we get \( Z = 5 \).
Step 3: Conclusion.
The nuclear charge \( Z \) is \( \boxed{5} \).
Quick Tip: To calculate the nuclear charge, use the Rydberg formula and solve for \( Z \). The longest wavelength corresponds to the transition between the n=1 and n=2 states.
Fullerene (C₆₀) crystallizes in an FCC unit cell (edge length = 14.14 Å) with one C₆₀ centered at each lattice point. The smallest distance (in Å) between the centers of two C₆₀ molecules is ..........
(Round off to two decimal places)
View Solution
Step 1: Understanding the FCC structure.
In a face-centered cubic (FCC) unit cell, the molecules are located at the corners and face centers of the unit cell. The distance between two centers of C₆₀ molecules corresponds to the edge length of the unit cell. The distance is the face diagonal for an FCC structure.
Step 2: Calculation.
The face diagonal \( d \) of an FCC unit cell is given by \( d = \sqrt{2}a \), where \( a \) is the edge length. Substituting \( a = 14.14 \, Å \), we get: \[ d = \sqrt{2} \times 14.14 = 20 \, Å \]
Thus, the smallest distance between two C₆₀ molecules is \( \boxed{20.00} \, Å \).
Quick Tip: In an FCC unit cell, the smallest distance between centers of molecules is the face diagonal, calculated as \( \sqrt{2}a \).
A film of stearic acid partially covers the water surface in a container. The work needed to decrease this coverage by 1 cm² is 25.0 × 10⁻⁷ J. The surface tension (in N/m) of the film is .......
(Round off to three decimal places)
(Surface tension of pure water is 0.072 N/m)
View Solution
Step 1: Understanding surface tension.
The work required to change the surface area of a liquid is related to the surface tension \( \gamma \) by: \[ W = \gamma \Delta A \]
Where \( W \) is the work, \( \Delta A \) is the change in area, and \( \gamma \) is the surface tension.
Step 2: Calculation.
Substitute \( W = 25.0 \times 10^{-7} \, J \) and \( \Delta A = 1 \, cm^2 = 1 \times 10^{-4} \, m^2 \): \[ \gamma = \frac{25.0 \times 10^{-7}}{1 \times 10^{-4}} = 0.025 \, N/m \]
Thus, the surface tension is \( \boxed{0.025} \, N/m \).
Quick Tip: Surface tension is calculated by dividing the work required to change the surface area by the change in area.
The value of ‘n’ in \([ P(O_3)_4 ]^{6-}\) is ............
View Solution
Step 1: Analyzing the structure.
The species \( [ P(O_3)_4 ]^{6-} \) indicates that the phosphorus is surrounded by four \( O_3 \) groups. The overall charge on the complex is \( 6- \), and phosphorus typically has a formal oxidation state of \( +5 \).
Step 2: Conclusion.
The value of \( n \) in this case is \( \boxed{5} \).
Quick Tip: For polyatomic ions, count the charge on the central atom and surrounding groups to determine oxidation states.
The total number of all possible isomers of \( [Co( H_2NCH_2CH_2NH_2)_2 ]^{2+} \) and \( [Co( H_2NCH_2CH_2NH_2)_3 ]^{3+} \) together is ............
View Solution
Step 1: Identifying the coordination geometry.
For both complexes, the coordination number of the central cobalt ion is 6. The ligands are ethylenediamine derivatives, each having two nitrogen donor atoms. The number of possible isomers depends on the different ways the ethylenediamine ligands can bind to the central metal.
Step 2: Analyzing the isomers.
- The \( [Co( H_2NCH_2CH_2NH_2)_2 ]^{2+} \) complex can have isomers due to different possible arrangements of the ethylenediamine ligands.
- The \( [Co( H_2NCH_2CH_2NH_2)_3 ]^{3+} \) complex can also have isomers due to ligand exchange and arrangement.
Step 3: Conclusion.
The total number of isomers is \( \boxed{6} \).
Quick Tip: For coordination complexes with ligands having multiple donor atoms, count the possible ways the ligands can be arranged around the central metal to determine the total number of isomers.
The number of lone pairs present in phosphonic acid (phosphorus acid) is .........
View Solution
Step 1: Structure of phosphonic acid.
Phosphonic acid has the structure \( H_3PO_3 \), where phosphorus is bonded to three hydroxyl groups and has one lone pair of electrons.
Step 2: Analyzing lone pairs.
Phosphorus in this case has one lone pair, and the oxygen atoms in the hydroxyl groups have two lone pairs each. The total number of lone pairs in the molecule is the sum of the lone pairs on phosphorus and oxygen atoms.
Step 3: Conclusion.
The total number of lone pairs is \( \boxed{4} \) (one on phosphorus and three on oxygen).
Quick Tip: When counting lone pairs, consider the bonding environment of each atom and the valency of the central atom.
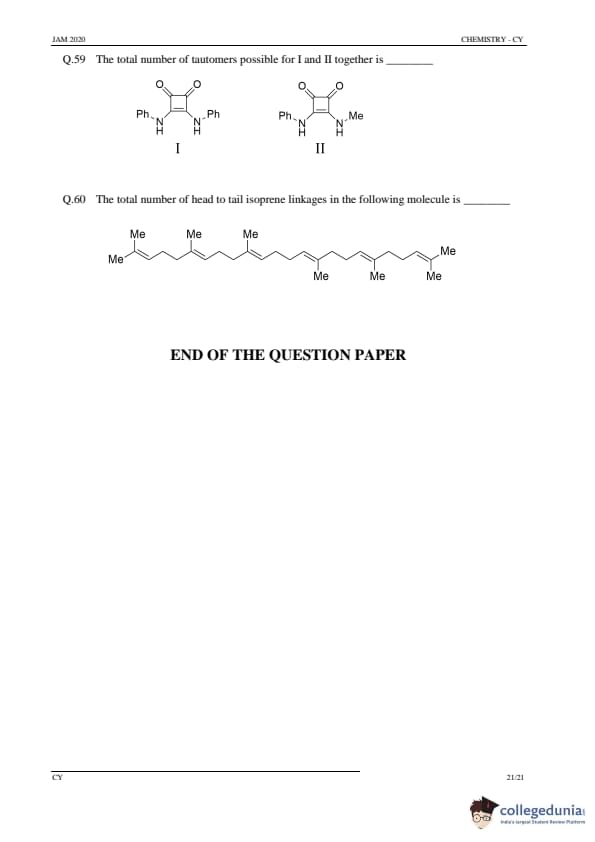
Total number of constitutional isomers possible for trimethyl cyclohexane is .......
View Solution
Step 1: Structure of trimethyl cyclohexane.
Trimethyl cyclohexane consists of a cyclohexane ring with three methyl groups attached at various positions.
Step 2: Analyzing the isomers.
- The methyl groups can be attached to different positions on the cyclohexane ring, leading to multiple constitutional isomers. By considering different possible locations for the methyl groups, there are 6 possible isomers.
Step 3: Conclusion.
The total number of constitutional isomers is \( \boxed{6} \).
Quick Tip: When determining the number of isomers, consider all possible ways the substituents can be arranged on the ring, taking into account symmetry and uniqueness of the resulting structures.
The dihedral (torsional) angle (in degrees) between the two methyl groups in the most stable conformation of n-butane is .......
(Round off to nearest integer)
View Solution
Step 1: Understanding the most stable conformation of n-butane.
In the most stable conformation of n-butane, the two methyl groups are positioned in the anti conformation, where the dihedral angle between them is maximized to reduce steric hindrance.
Step 2: Analyzing the torsional angle.
In the anti conformation, the dihedral angle between the two methyl groups is 180°.
Step 3: Conclusion.
The dihedral angle is \( \boxed{180^\circ} \).
Quick Tip: In the most stable conformation of butane (the anti conformation), the dihedral angle between the two methyl groups is 180°.
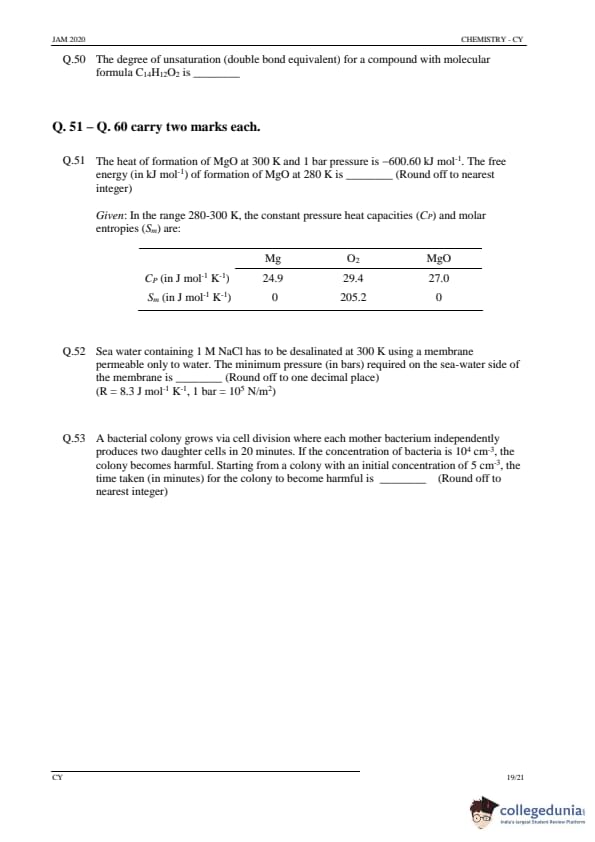
The degree of unsaturation (double bond equivalent) for a compound with molecular formula \( C_{14}H_{12}O_2 \) is .......
View Solution
Step 1: Formula for degree of unsaturation.
The degree of unsaturation (DU) is calculated using the formula: \[ DU = \frac{2C + 2 - H}{2} \]
Where \( C \) is the number of carbons and \( H \) is the number of hydrogens.
Step 2: Calculation.
For \( C_{14}H_{12}O_2 \), substituting into the formula: \[ DU = \frac{2(14) + 2 - 12}{2} = \frac{28 + 2 - 12}{2} = \frac{18}{2} = 9 \]
Step 3: Conclusion.
The degree of unsaturation is \( \boxed{9} \).
Quick Tip: The degree of unsaturation tells you the number of rings and double bonds in a molecule. Calculate it using the formula \( \frac{2C + 2 - H}{2} \).





IIT JAM Previous Year Question Papers
| IIT JAM 2022 Question Papers | IIT JAM 2021 Question Papers | IIT JAM 2020 Question Papers |
| IIT JAM 2019 Question Papers | IIT JAM 2018 Question Papers | IIT JAM Practice Papers |



Comments