Muskan Shafi Education Content Expert
Education Content Expert
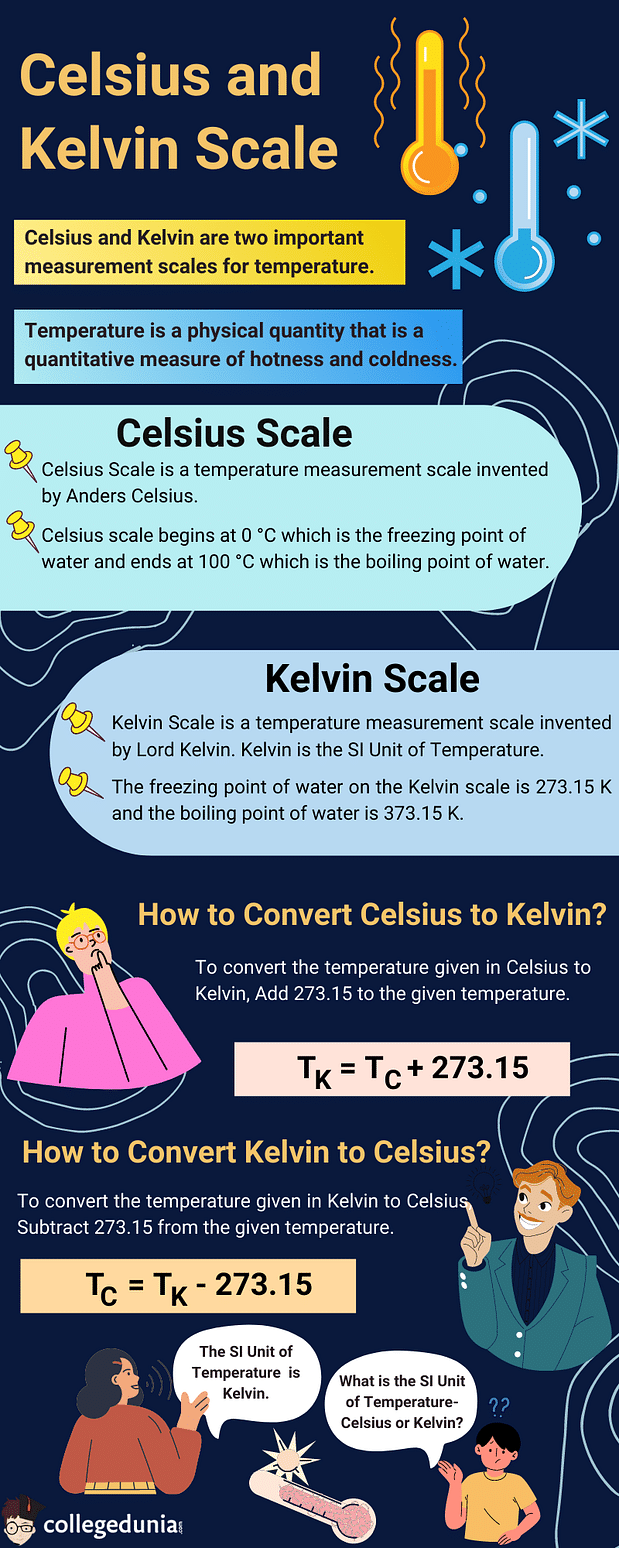
Celsius and Kelvin are two important measurement scales for temperature. Temperature is a physical quantity that is a quantitative measure of hotness and coldness.
- Kelvin (K) is the official base unit of temperature in the SI system.
- Celsius (oC) is the most commonly used scale for temperature in the SI system.
- Temperature in Celsius is represented in ‘°C’ while it is represented in ‘K’ in the Kelvin scale.
The conversion of temperature from Celsius to Kevin can be done with the help of the given formula
| TK = TC + 273.15 |
Here TK is the temperature in Kelvin and TC is the temperature in Celsius.
Read More: Kelvin to Celsius
Key Terms: Celsius, Kelvin, Absolute Zero Temperature, Celsius to Kelvin Formula, Temperature, Boiling Point, Freezing Point
What is Celsius?
[Click Here for Sample Questions]
Celsius Scale is a temperature measurement scale invented by Anders Celsius.
- The temperature on the Celsius scale is written as °C.
- It is also known as the Centigrade Scale.
- This scale is split into 100 equal divisions called degrees.
- Celsius scale begins at 0o C which is the freezing point of water and ends at 100oC that is the boiling point of water.
- The scale is also extended for including negative numbers.

Celsius scale
Read More:
What is Kelvin?
[Click Here for Previous Years’ Questions]
Kelvin Scale was invented by Lord Kelvin.
- The unit Kelvin (K) is the SI unit of temperature as per the International System of Units.
- The fraction 1/273.16 of the thermodynamic temperature illustrates the triple point of water.
- It divides the interval between the points of ice and steam into 100 divisions so that one kelvin represents the same temperature interval as one °C.
- Kelvin is represented without the symbol (°) as it is an absolute scale based on absolute zero.
- This scale does not have negative values.

Kelvin Scale
Read More: NCERT Solutions For Class 11 Physics Thermal Properties of Matter
Celsius to Kelvin Formula
[Click Here for Sample Questions]
Celsius to Kelvin Formula is used to convert the unit of temperature from °C to K. In order to convert temperature from Celcius to Kelvin, add the number 273.15 to it. In simpler terms, the temperature in Kelvin (K) is equal to the temperature in degrees Celsius (°C) plus 273.15.
The Celsius to Kelvin Formula is given as follows:
| TK = TC + 273.15 |
Where
- TK: Temperature in Kelvin
- TC: Temperature in Celsius
Read More: Fahrenheit to Celsius Formula
Solved ExamplesExample 1: What will be the value of 15°C in Kelvin? Solution: The temperature in Celsius is given as 15°C. Using the Celsius to Kelvin formula, TK = TC + 273.15 TK = 15 + 273.15 = 288.15 K Thus, 15°C is equal to 288.15 K on the Kelvin scale. Example 2: Convert 312°C in Kelvin. Solution: The given temperature in Celsius is 312°C. Using the Celsius to Kelvin formula, TK = TC + 273.15 TK = 312 + 273.15 = 585.15 K Thus, 312°C is equal to 585.15 K on the Kelvin scale. |
Celsius to Kelvin Formula Derivation
[Click Here for Previous Years’ Questions]
The temperature on the Kelvin scale is exactly 273.15 more than the corresponding temperature on the Celsius scale. We can convert a given temperature in Kelvin by adding 273.15 to the temperature in the Celsius scale.
The freezing point and the boiling point of water in both Celsius and Kelvin scales are as follows:
| Reference Point | Celsius Scale | Kelvin Scale |
|---|---|---|
| Freezing Point | 0°C | 273.15 K |
| Boiling Point | 100°C | 373.15 K |
Read More: Thermal Properties of Matter Important Questions
Celsius and Kelvin Scale
Celsius to Kelvin Conversion Table
[Click Here for Sample Questions]
The table given below lists the values of some common reference points in both Kevin and Degree Celsius.
| Reference Point | Kelvin | Celsius |
|---|---|---|
| Boiling Point of Water | 373 K | 100 oC |
| Body Temperature | 310 K | 37 oC |
| Room Temperature | 293 K | 20 oC |
| Freezing Point of Water | 273 K | 0 oC |
| Absolute Zero | 0 K | -273 oC |
Absolute Zero Temperature
[Click Here for Previous Years’ Questions]
At Absolute Zero Temperature, the particles cool down and tend to cease movements.
- They have zero kinetic energy at this temperature.
- As cooling down the temperature beyond a limit only increases the energy, scientists are unable to bring the absolute zero temperature into reality.

Absolute Zero Temperature
Check More:
Things to Remember
- Celsius and Kelvin are two scales used for the measurement of temperature.
- Celsius Scale was invented by Anders Celsius while the Kelvin scale was invented by Lord Kelvin.
- Temperature is expressed as °C on the Celsius scale while it is represented as K on the Kelvin scale.
- Celsius to Kelvin conversion is done using the formula TK = TC + 273.15.
- Kelvin to Celsius conversion is done using the formula TC = TK - 273.15.
- There is no temperature where Celsius and Kelvin are equal.
- Celsius is more practical, thus, it is more popularly used in laboratories.
Previous Years’ Questions
- Absolute zero is the condition at which… [AFMC 2003]
- The absolute zero is the temperature at which…
- A pan filled with hot food cools from 94°C to 86°C in… [KCET 2016]
- A dark pattern on a China dish appears brighter…..[KCET 1997]
- A hollow metallic cylinder is heated. Then its internal diameter... [KCET 1994]
- A bimetallic strip of brass and iron is heated... [KCET 1994]
- A ring-shaped metal is allowed to expand by heating it. The hole will... [KCET 1991]
- Absolute zero of temperature is... [KCET 1990]
- Blowing air with an open mouth is an example of... [KCET 2004]
- Device used to measure very high temperature is... [KCET 1998]
- Hot water cools from 60°C to 50°C... [KCET 2010]
- In an anomalous expansion of water… [KCET 2014]
Sample Questions
Ques. Convert the following temperature from Celsius to Kelvin.
a. 20 oC
b. 76 oC
c. - 48 oC
d. 134 oC
e. 236 oC (5 Marks)
Ans. The conversion from celsius to kelvin is as follows:
a. 20oC
K = oC + 273.15 = 20 + 273.15
20oC = 293.15 K
b. 76oC
K = oC + 273.15 = 76 + 273.15
76oC = 349.15 K
c. - 48oC
K = oC + 273.15 = - 48 + 273.15
-48oC = 225 K
d. 134oC
K = oC + 273.15 = 134 + 273.15
134oC = 407.15 K
e. 236oC
K = oC + 273.15 = 236 + 273.15
236oC = 509.15 K
Ques. Convert the following temperature from Kelvin to Celsius.
a. 300 K
b. 437 K
c. 102 K
d. 30 K
e. 272 K (2 Marks)
Ans. The conversion from Kelvin to Celsius is as follows:
a. 300 K
oC = K – 273.15 = 300 – 273.15
300 K = 27.15oC
b. 437 K
oC = K – 273.15 = 437 – 273.15
437 K = 164.15oC
c. 102 K
oC = K – 273.15 = 102 – 273.15
102 K = -171.15oC
d. 30 K
oC = K – 273.15 = 30 – 273.15
30 K = -243.15oC
e. 272 K
oC = K – 273.15 = 272 – 273.15
272 K = -1.15oC
Ques. Isopropyl alcohol boils at 82.5oC and freezes at -89oC under a pressure of 1 atm. Convert these temperatures to the Kelvin scale. (2 Marks)
Ans. The boiling point of Isopropyl alcohol is 82.5oC thus in Kelvin,
= 82.5 + 273.15
= 355.65 K
Freezing point of Isopropyl alcohol is -89oC thus in Kelvin,
= -89 + 273.15
= 184.15 K
Ques. Which scale is more preferred- Kelvin or Celsius? (2 Marks)
Ans. Kelvin is a more reliable unit of temperature as its measurement is directly proportional to the pressure and volume at constant moles of gas whereas measuring in Celsius doesn’t give the same results. Thus, by indicating a proportional relationship between pressure and volume, Kelvin Temperatures report the kinetic energy (thermal energy) directly in the system. This makes it important to convert Celsius to Kelvin while solving scientifically sensitive problems.
Ques. Mercury’s freezing point is 234.32 K. Which state will it be found at room temperature of 25oC? (2 Marks)
Ans. Let us first convert the freezing point of mercury which is in Kelvin into Celsius to compare it with the given room temperature.
= K – 273.15
= 234.32 – 273.15
= - 38.83 oC
Thus the freezing point of mercury is - 38.83oC which is lower than the given room temperature of 25oC. Thus it will be in a liquid state.
Ques. Give reasons why Kelvin Scale is better than Celsius. (3 Marks)
Ans. The reasons why Kelvin is preferred more than Celsius are:
- According to the Ideal Gas Law, Pressure and Volume are directly proportional to the temperature. Thus the temperature of a gas will be different at different pressures. Kelvin’s absolute scale is used as its temperature measurement is based on the kinetic energy of the gas and is irrespective of the pressure.
- Thermodynamically, Kelvin directly expresses the volume and kinetic energy of the gas.
- If you double 30 oC to 60 oC the thermal energy of the gas will not be doubled, ultimately it doesn’t have any meaning thermodynamically. To simplify, if you double -30 oC, will you double it to -60oC or -10 oC. This confusion is avoided while using Kelvin.
- Kelvin scale does not use negative values in its scale, thus extremely hot or cold temperatures can be expressed in a positive value. This is useful to avoid anomalies while solving problems using these temperatures.
- Kelvin is used to measure the color and noise temperature.
Ques. How to convert Celsius to Kelvin? (2 Marks)
Ans. In order to convert a temperature in Celcius to Kelvin, we need to use the given formula,
Kelvin = Celsius + 273.15.
Sometimes, instead of 273.15, the value 273 is also used.
Ques. What is 0 oC in Kelvin? (2 Marks)
Ans. Using the Celsius to Kelvin formula, we need to add 273.15 to 0 to get the temperature in Kelvin. Thus,
0°C = 0 + 273.15 = 273.15 K.
Therefore, 0 oC in Kelvin is equal to 273.15 K.
Ques. The boiling point of water is 100°C. What will be the same in Kelvin? (2 Marks)
Ans. It is given that
- TC = 100°C
- TK =?
Using the Celsius to Kelvin formula,
TK = TC + 273.15
TK = 100 + 273.15
TK = 373.15 K
Thus, the boiling point of water in Kelvin is 373.15 K.
Ques. What is the main difference between Celsius and Kelvin scales? (2 Marks)
Ans. Celsius and Kelvin scales are used for the measurement of temperature. The main difference between Celsius and Kelvin scales is the zero point. The zero point on the kelvin scale is the theoretical temperature indicating no further heat loss, which is absolute zero. When Celsius is converted to Kelvin, the Kelvin measurement will be larger than the Celsius measurement.
Check-Out:




Comments