TS EAMCET 2024 Question Paper May 8 Shift 1 with Answer Key PDF is available here for download. JNTU, Hyderabad on behalf of TSCHE conducted TS EAMCET on May 8 from 9 AM to 12 PM. TS EAMCET 2024 Question Paper consists of 160 questions carrying 1 mark each. TS EAMCET 2024 Question Paper May 8 Shift 1 PDF for BiPC includes four subjects, Physics, Chemistry and Biology with Botany & Zoology. Each subject includes 40 questions.
TS EAMCET 2024 Question Paper with Answer Key May 8 Shift 1 PDF
| TS EAMCET 2024 Question Paper with Answer Key | Check Solution |
In the following group of plants, sporophytes are dependent on gametophytes.
View Solution
Assertion (A): Endosperm is haploid in Gymnosperms
Reason (R): Female gametophytic tissue acts as endosperm in Gymnosperms
View Solution
Assertion (A): Ascospores are produced endogenously in ascus
Reason (R): Basidiospores are produced exogenously on the basidium
View Solution
The assertion about Ascospores being produced endogenously is correct. Ascospores develop inside the ascus. However, the reason given about Basidiospores being produced exogenously on the basidium is also correct but is not related to the assertion, as Basidiospores are associated with basidiomycetes, not ascomycetes. Quick Tip: The assertion and reason are true, but they describe different processes: Ascospores in Ascomycetes and Basidiospores in Basidiomycetes.
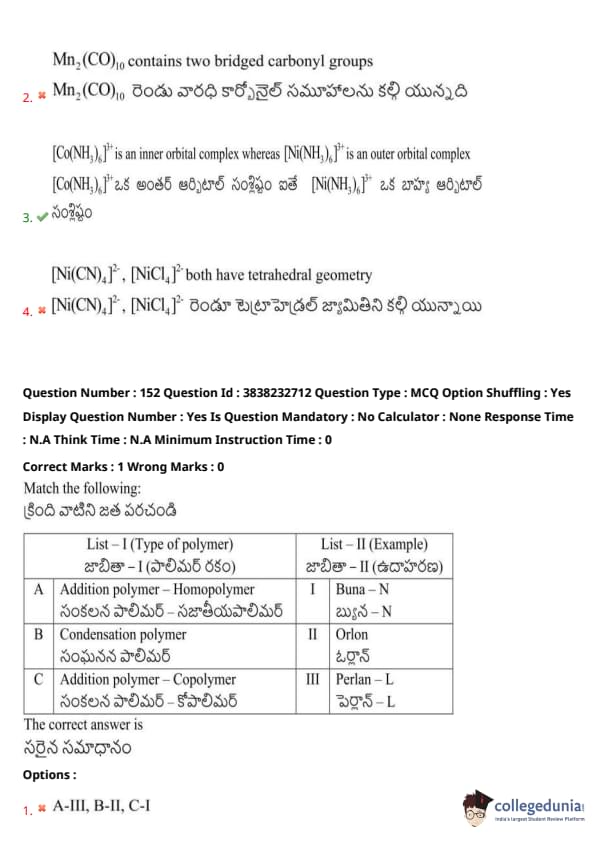
Match the following:
View Solution
The number of stamens found in a single male flower of Cyathium inflorescence is:
View Solution
‘Pepo’ fruit develops from the following type of ovary:
View Solution
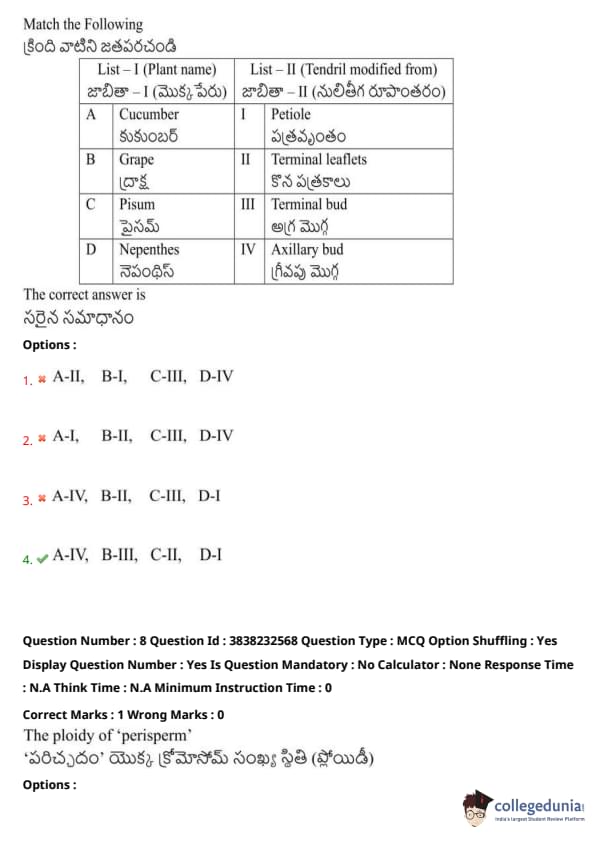
Match the following:
List - I (Plant name) \hspace{1cm List - II (Tendril modified from)
View Solution
The ploidy of 'perisperm':
View Solution
Intine of pollen grain is made up of:
View Solution
The ratio of microspore mother cell to male gametes in a typical angiospermic plant:
View Solution
"Families of flowering plants" book was written by:
View Solution
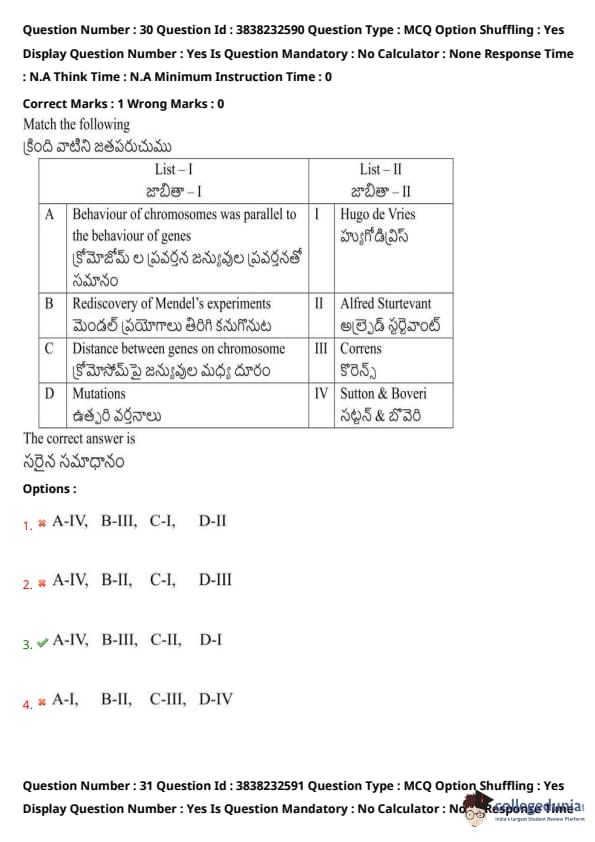
Match the following:
View Solution
The following type of ribosome sub-units are present in an eukaryotic cell:
View Solution
The site of t-RNA synthesis:
View Solution
DNA molecule having the length of 68Å, contains 10% Adenine. How many number of hydrogen bonds are present between nitrogen bases totally in that DNA?
View Solution
Assertion (A): Crossing-over leads to genetic recombinations.
Reason (R): Crossing-over is the exchange of chromatin bits between two sister chromatids of homologous chromosomes.
View Solution
- The assertion (A) is correct because crossing-over during meiosis results in the exchange of genetic material between homologous chromosomes, leading to genetic recombination. This is a key mechanism that increases genetic diversity.
- The reason (R), however, is not entirely accurate. Crossing-over occurs between non-sister chromatids of homologous chromosomes, not between the sister chromatids of the same chromosome. Sister chromatids are identical and don't undergo the exchange of genetic material during crossing-over.
Thus, while the assertion is correct, the reason is incorrect, and the correct explanation of crossing-over involves the exchange between non-sister chromatids, not sister chromatids. Quick Tip: Crossing-over occurs between non-sister chromatids of homologous chromosomes during meiosis, resulting in genetic recombination and diversity.
The following type of cell divisions occurs in microspore mother cells to increase their number in microsporangium:
View Solution
The following characters are found common to both mitochondria and chloroplast:
View Solution
Lateral roots develop (originate) from the following tissue:
View Solution
Among the following the most important pollinator for agricultural purposes is:
View Solution
The following is not a microelement:
View Solution
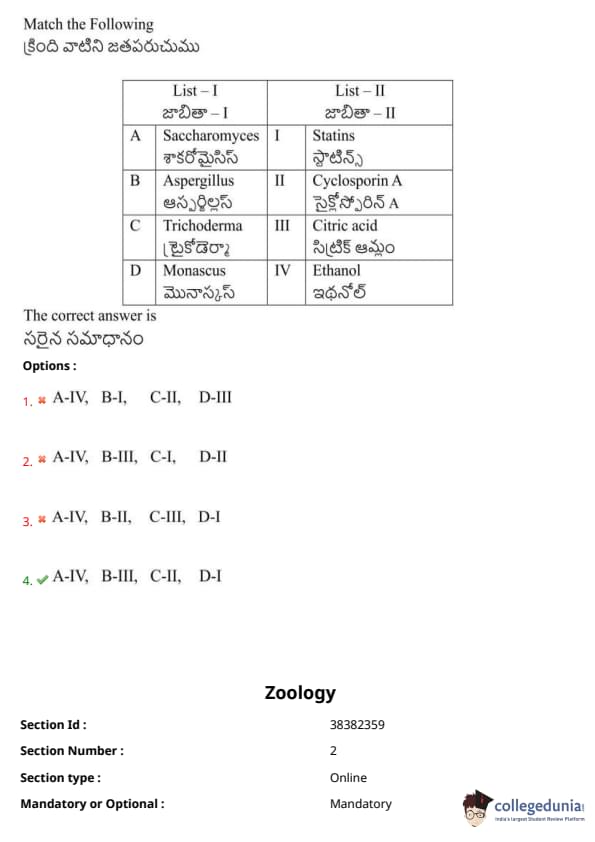
Match the following:
View Solution
The first reaction in photosynthesis is:
View Solution
Find out the correct statements among the following:
A. In C4 plants photosrespiration is absent.
B. In the photorespiration pathway, there is no synthesis of ATP and NADPH.
C. C4 plants are photosynthetically more efficient plants.
D. Kranz anatomy is found in C4 and CAM plants.
View Solution
During the process of aerobic respiration, O\(_2\) utilisation occurs in the following stage only:
View Solution
The final acceptor of e\(^{-}\) (electrons) during non-cyclic photophosphorylation is:
View Solution
In Phloem, the food material is mostly translocated in the following form:
View Solution
Cytochrome ‘C’ transfers the electrons between:
View Solution
The type of nucleic acid found in a virus:
View Solution
Match the following:
View Solution
If a heterozygous tall plant is crossed with a homozygous dwarf plant, the percentage of progeny having dwarf character is:
View Solution
The percentage of 'ab' genotype gametes produced by ‘AaBb’ parent plant is:
View Solution
Find out the correct statements among the following:
A. DNA is (-Ve) charged, Histone proteins are (+Ve) charged.
B. DNA is (+Ve) charged, Histone proteins are (-Ve) charged.
C. Euchromatin is transcriptionally active.
D. Euchromatin and heterochromatin both are transcriptionally inactive.
View Solution
The haploid content of human DNA contains:
View Solution
UUU, CCC, AAA, GGG are the codons codes for the following amino acids respectively:
View Solution
Identify the correct statements among the following:
A. RNA interference (RNAi) takes place in all eukaryotic organisms as a method of cellular defense.
B. Bt-toxin gene, CryIIAb controls cotton bollworms.
C. Mycorrhiza helps the plants for more absorption of potassium.
D. Elusion is a technique of extracting separated bands of DNA from agarose gel.
View Solution
Match the following:
List - I (Item) \hspace{1cm List - II (Description)
View Solution
Identify the correct statements among the following:
A. Probes are generally ss RNA or ss DNA
B. Probes are complementary to desired DNA
C. The efficiency of uptaking DNA fragments by bacteria decreases when they are treated with Ca\(^{2+}\) ions
D. Cloning vectors should have low molecular weight
View Solution
Use of microbes to remove the toxic (waste) substances that are released into the environment is:
View Solution
Match the following:
View Solution
Study the following and pick up the correct statements:
I. Greater biodiversity is found in temperate regions
II. Invasion of alien species is a threat for local species
III. Loris tardigradus is one of the threatened species in our country
IV. A biographic region with significant reservoir of biodiversity that is under threat of extinction from humans is called a sanctuary
View Solution
Species is considered as a group of individuals which are showing similarity in karyote. Hence, species is a
View Solution
Assertion (A): Most of the cranial bones are dermal bones
Reason (R): They are formed by the ossification in the embryonic mesenchyme
View Solution
The assertion states that "most of the cranial bones are dermal bones," which is true. Dermal bones are those bones that form directly from mesenchymal tissue through a process called intramembranous ossification. Cranial bones such as the flat bones of the skull are formed in this way.
The reason states that "they are formed by the ossification in the embryonic mesenchyme," which is also true. Dermal bones develop from mesenchyme (embryonic connective tissue) through ossification, and this process happens without a cartilage stage, which is typical of endochondral ossification seen in other bones.
Thus, both (A) and (R) are correct, and (R) is the correct explanation of (A). Quick Tip: Dermal bones are commonly found in the skull, such as in the parietal, frontal, and occipital bones, which develop through intramembranous ossification.
Match the following:
View Solution
Pseudocoelom is the body cavity of:
View Solution
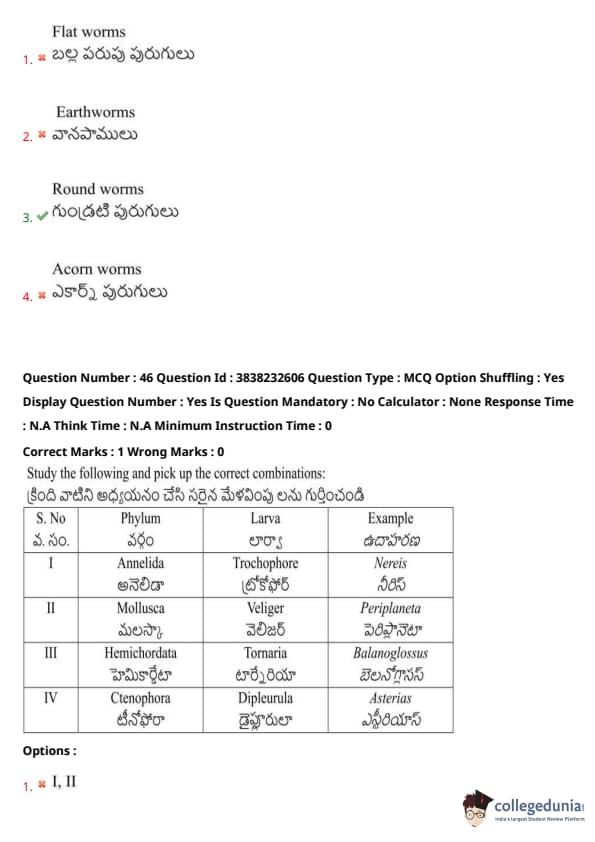
Study the following and pick up the correct combinations:
View Solution
Molluscs having closed circulatory system are included in the class
View Solution
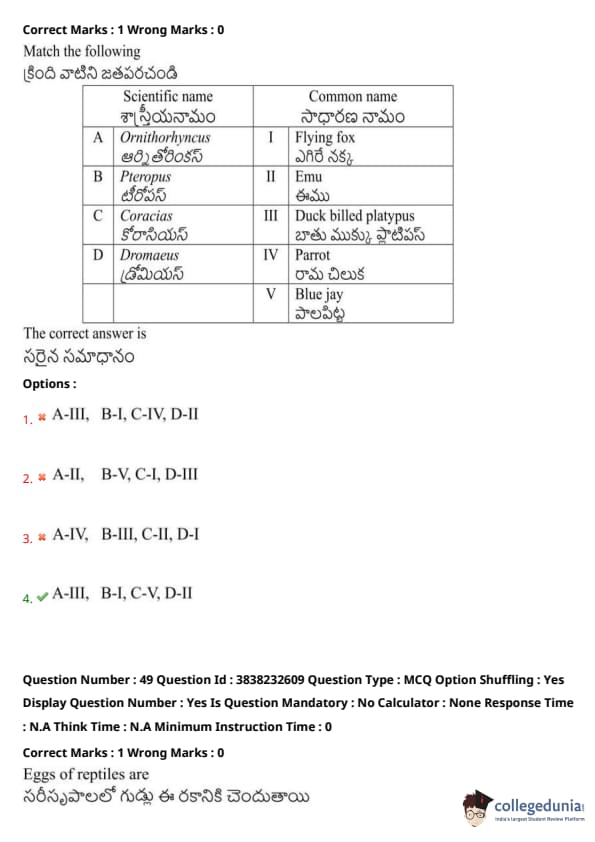
Match the following:
View Solution
Eggs of reptiles are
View Solution
Flagellum in Polytoma is
View Solution
Study the following and pick up the correct statements:
I. Opioids are obtained from \textit{Cannabis sativa
II. Heroin is obtained by the acetylation of Morphine
III. Barbiturates cause sleeplessness
IV. Benzodiazepines are tranquilizers
View Solution
Assertion (A): Syngamy in \textit{Plasmodium is anisogamy
Reason (R): The gametes are similar in size
The correct answer is: Assertion (A) and Reason (R) are correct, but (R) is not the correct explanation of (A).
View Solution
Parasite responsible for hyperplasia in its host is:
View Solution
Benign tertian malaria is caused by:
View Solution
These cells of cockroach contain symbiotic bacteria:
View Solution
In cockroach, egg case is secreted by these glands:
View Solution
In cockroach, thermoreceptor sensillae lie:
View Solution
This rule states that with the increase of every 10° C, the rate of metabolic activities double:
View Solution
Statement I: The animals that are capable of swimming in water are known as neuston.
Statement II: Decomposers are absent in limnetic zone.
View Solution
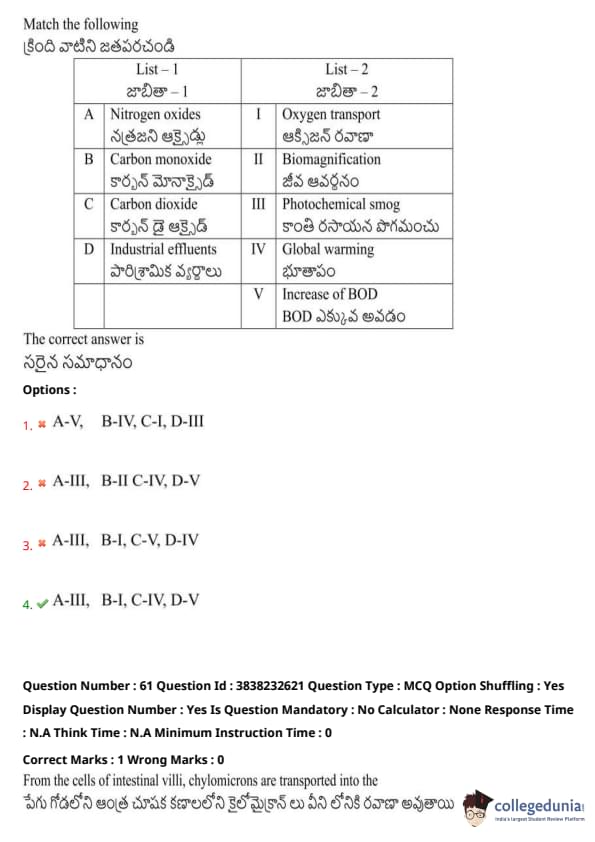
Match the following:
View Solution
From the cells of intestinal villi, chylomicrons are transported into the:
View Solution
Assertion (A): Female human beings produce a high pitch voice.
Reason (R): The vocal cords in females are long and thick.
View Solution
Statement I: Right atrio ventricular aperture in human heart is guarded by a tricuspid valve.
Statement II: Blood plasma without fibrinogen and some other plasma proteins is called lymph.
View Solution
These structures eliminate sterols, hydrocarbons, waxes etc. in human beings:
View Solution
Dentalveolar joint is an example for:
View Solution
Study the following and pick up the correct statements:
I. Superior colliculi are concerned with auditory function
II. Inferior colliculi are concerned with visual function
III. Pneumotaxic centre lies in pons varoli
IV. Thermoregulatory centre lies in the hypothalamus
View Solution
Study the following and pick up the correct combinations:
View Solution
These hormones are commonly called catecholamines:
View Solution
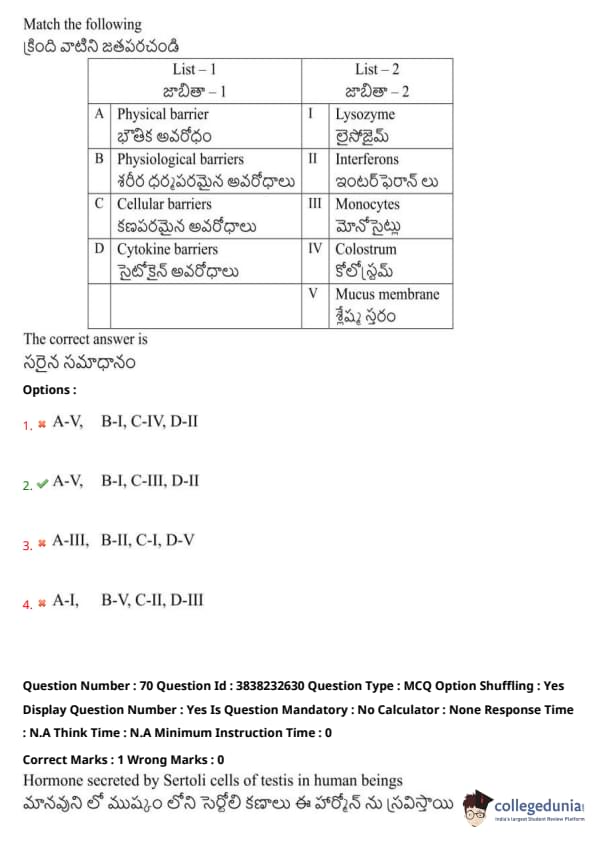
Match the following:
View Solution
Hormone secreted by Sertoli cells of testes in human beings:
View Solution
Pick up the incorrect pair:
View Solution
If a red eyed male Drosophila and white eyed female Drosophila are crossed, the eye color in their offspring is:
View Solution
A person is with short stature, small round head, furrowed tongue and partially open mouth and retarded physical, psychomotor and mental development. The person is said to be suffering from this genetic disorder:
View Solution
In human genome, the highest number of genes are present in:
View Solution
Cynognathus is a transitional form between:
View Solution
Statement I: Jean Baptiste de Lamarck stated that acquired characters are inherited to the next generations.
Statement II: August Weismann strongly supported the view of inheritance of acquired characters.
View Solution
If one species diverges to become two or more species, it is called:
View Solution
Assertion (A): Magnetic Resonance Imaging is generally a very safe procedure.
Reason (R): It does not use ionising radiation.
View Solution
Prolonged P-R interval in ECG indicates:
View Solution
Match the following:
List-1:
A Direct ELISA
B Indirect ELISA
C EEG
D CAT
List-2:
I Antibodies
II Brain
III Antigens
IV Tomogram
V Heart
View Solution
When two resistors of resistances \( (123 \pm 2) \, \Omega \) and \( (227 \pm 4) \, \Omega \) are connected in series, then the value of equivalent resistance is:
View Solution
- When resistors are connected in series, their resistances add up. The equivalent resistance \( R_{eq} \) is given by:
\[ R_{eq} = R_1 + R_2 = (123 \, \Omega + 227 \, \Omega) \pm (2 \, \Omega + 4 \, \Omega) = 350 \, \Omega \pm 6 \, \Omega \]
- The uncertainty in the resistance is the sum of the individual uncertainties, i.e., \( 2 + 4 = 6 \). Quick Tip: In a series connection, the equivalent resistance is the sum of the individual resistances, and the uncertainty is the sum of their uncertainties.
Starting from rest, a car accelerates with uniform acceleration of \( 4 \, m/s^2 \) for some time after which it comes to rest with uniform deceleration of \( 6 \, m/s^2 \). If the total time of travel is 5 s, then the total distance travelled by the car is:
View Solution
Two bodies A and B are projected simultaneously with velocities \( 20 \, ms^{-1} \) and \( 40 \, ms^{-1} \), respectively. Body A is projected vertically up from the top of a tower of height 80 m and body B is projected vertically up from the bottom of the same tower. The bodies A and B meet in time of:
View Solution
- For body A, which is projected upwards, use the equation of motion:
\[ h_A = v_0 t + \frac{1}{2} (-g) t^2 \]
where \( h_A = 80 \, m \), \( v_0 = 20 \, ms^{-1} \), and \( g = 10 \, ms^{-2} \).
- For body B, the motion is also vertical but downwards, and it will follow:
\[ h_B = v_0 t + \frac{1}{2} g t^2 \]
where \( v_0 = 40 \, ms^{-1} \).
- Solving these two equations for the time \( t \), the bodies will meet after 4 seconds. Quick Tip: For two bodies moving towards each other, solve the equations of motion for each body and find when their displacements add up to the total distance.
A body is projected from the surface of the earth with a velocity of \( 10 \sqrt{3} \, m/s \) such that its range is maximum. The velocity of the body at half of the maximum height is:
View Solution
A body of mass 10 kg is kept on a rough horizontal surface. If the force applied on the body is increased by 80 N, then the acceleration of the body increases by:
View Solution
A block enters a rough horizontal surface with a speed of \( 6 \, ms^{-1} \) at \( x = 1.5 \, m \) and leaves the rough horizontal surface with a speed of \( 4 \, ms^{-1} \) at \( x = 2.5 \, m \). If the retarding force acting on the block is \( F = -25x \, N \) (where \( F \) is in newton and \( x \) is in meter), then the mass of the block is:
View Solution
A body of mass 3 kg is thrown vertically upward from the ground with a velocity of \( 10 \, ms^{-1} \). If the maximum height reached by the body is 4.7 m, then the loss of energy due to air resistance is:
View Solution
Four bodies of masses 8 kg, 2 kg, 4 kg and 2 kg are placed at the four corners A, B, C and D respectively of a square ABCD of diagonal 80 cm. The distance of the center of mass of the system from the corner A is:
View Solution
A thin circular ring of mass 0.2 kg is rotating about its axis with an angular speed of 51 rad/s. Two particles having mass 2 g each are now attached at diametrically opposite points on the ring. Then the angular speed of the system is:
View Solution
The amplitude of a damped harmonic oscillator becomes \( \frac{1}{n} \) times its initial amplitude \( A_0 \) at the end of 20 oscillations. The amplitude of the oscillator when it completes 40 oscillations is:
View Solution
A body weighs the same on the surfaces of two planets of densities \( p_1 \) and \( p_2 \). The ratio of the radii of the planets is:
View Solution
A copper wire of cross-sectional area 0.01 cm\(^2\) is subjected to a tension of 22 N. If Young’s modulus and Poisson’s ratio of copper are \(1.1 \times 10^{11}\) Nm\(^2\) and 0.32 respectively, then the change in the cross-sectional area of the wire is:
View Solution
A soap bubble is given a negative charge. The pressure inside the bubble:
View Solution
A wooden cube is floating in a bucket of water with \(\frac{3}{4}\) of its volume immersed. If this bucket with the wooden block is now placed in a lift moving down with an acceleration of \(\frac{g}{2}\), the fraction of volume of the wooden cube immersed in water is:
View Solution
The initial and the final temperatures of a black body are \(27^\circ C\) and \(177^\circ C\) respectively. The increase in the amount of radiation emitted per second is:
View Solution
The change in moment of inertia of a solid sphere of mass \( M \), radius \( R \), for a small change in temperature \( \Delta t \) is:
View Solution
The coefficient of performance of a refrigerator is 5. If it is placed in a room at a temperature of 39°C, the temperature inside the refrigerator is:
View Solution
The ratio of the degrees of freedom of monatomic and diatomic gas molecules is:
View Solution
An observer is standing below a freely falling source of sound of frequency 900 Hz. The change in the frequency noticed by the observer after 3 seconds of free fall of the source is (speed of sound in air is 330 ms\(^{-1}\) and acceleration due to gravity = 10 ms\(^{-2}\))
View Solution
An observer is standing below a freely falling source of sound of frequency 900 Hz. The change in the frequency noticed by the observer after 3 seconds of free fall of the source is (speed of sound in air is 330 ms\(^{-1}\) and acceleration due to gravity = 10 ms\(^{-2}\))
View Solution
A convex lens of focal length 10 cm is placed coaxially at a distance of 4 cm to the right of another convex lens of focal length 16 cm. If an object is placed at a distance of 8 cm to the left of the convex lens of focal length 16 cm, then the distance of the final image from the object is:
View Solution
A prism having angle \(\theta\) and refractive index of the material of prism ‘n’ are related by
\[ \theta = 2\sin^{-1} \left( \frac{1}{\sqrt{n^2 + 1}} \right) \]
- If the angle of minimum deviation is \(60^\circ\), then the angle of the prism is
View Solution
A polaroid sheet ‘P’ is placed on another similar polaroid sheet ‘Q’ such that the angle between their axes is \(45^\circ\).
- The ratio of the intensities of the light emerged from polaroid ‘Q’ and the unpolarised light incident on polaroid ‘P’ is
View Solution
A charged particle of mass 5 g and charge 20 μC is thrown with a velocity of 16 m/s\(^-1\) in a direction opposite to the direction of a uniform electric field of \( 2 \times 10^5 \) N/C. The distance travelled by the particle before coming to rest is:
View Solution
Two positive point charges of \(10 \, \mu C\) and \(12 \, \mu C\) are kept in air with a separation of 12 cm.
- To make the distance between the charges 4 cm, the work done is
View Solution
A storage battery of emf \(10V\) and internal resistance \(1 \Omega\) is being charged by a \(100V\) dc supply using a series resistor of \(17 \Omega\).
- The terminal voltage of the battery during charging is
View Solution
In a meter bridge, when an unknown resistance ‘R’ is connected in the left gap, the null point is obtained at 25 cm from the left end of the wire.
- If the resistance in the left gap is increased by 100%, the distance of the null point from the left end of the wire increases by
View Solution
An electron moving with a velocity of \(4.8 \times 10^6 \, ms^{-1}\) enters a uniform magnetic field of \(0.182 \, T\) in a direction perpendicular to the field.
- The radius of the circular path in which the electron moves under the influence of the magnetic field is
- (Mass of electron \(= 9.1 \times 10^{-31} \, kg\) and charge of electron \(= 1.6 \times 10^{-19} \, C\))
View Solution
A long solenoid of length \(75\) cm carries a current of \(3.5\) A. If the number of turns of the solenoid is \(600\), the magnitude of the magnetic field inside the solenoid is
View Solution
A coil of 200 turns and area of cross-section \(5 \times 10^{-3} \, m^2\) carries a current of 3 A.
- The magnetic moment of the coil is
View Solution
When a diamagnetic substance of relative permeability \(0.5\) is filled inside a solenoid, then its self-inductance
View Solution
When an alternating voltage given by \(E = 100 \sin(10^2 t)\), where \(E\) is in volts and \(t\) is in seconds, is applied across a capacitor of capacitance \(20 \, \mu F\), the peak current flowing in the circuit is
View Solution
If two electromagnetic waves with electric fields given by
\[ \vec{E_1} = E_0 \sin (kx - \omega t) \hat{j} \]
and
\[ \vec{E_2} = E_0 \sin (kx - \omega t + \pi) \hat{j} \]
- interfere, then the peak value of the electric field of the resultant wave is
View Solution
If the wavelength of the incident radiation on a photosensitive metal surface is decreased from \(3100 \, Å\) to \(1550 \, Å\), the maximum kinetic energy of the emitted photoelectrons is tripled.
- The work function of the metal surface is
View Solution
In Geiger-Marsden experiment, if the initial speed of \(\alpha\) particle is doubled, then the closest distance of approach of the \(\alpha\) particle from the gold nucleus
- (Assume \(\alpha\) particle is projected straight towards the gold nucleus)
View Solution
The energy equivalent of \(3.2 \, \mu g\) of mass is
View Solution
If the nucleus \(X^{240}\), initially at rest, splits into two daughter nuclei \(Y^{100}\) and \(Z^{140}\), then the ratio of the kinetic energies of \(Y\) and \(Z\) is
View Solution
In a common emitter transistor circuit, if the emitter current is changed by 4 mA, the collector current changes by 3.5 mA, then the current gain is
View Solution
The power delivered by the battery shown in the figure is
- (Take the forward and reverse biased resistances of the diode are zero and infinity respectively)
View Solution
A \(5\) kHz frequency signal is amplitude modulated on a carrier wave of frequency \(2\) MHz.
- One possible frequency of the resultant signal is
View Solution
The momentum of an electron is \(6.625 \times 10^{-28} \, kg \cdot ms^{-1}\). What is its wavelength in nm?
- (Given: \( h = 6.625 \times 10^{-34} \, J \cdot s\))
View Solution
The following orbital energies (\(E\)) are compared. Identify the correct sets
(I) \quad E_2(H) = E_2(H)
(II) \quad E_3(H) = E_3(He)
(III) \quad E_{2s(H) < E_{2s(He)
(IV) \quad E_{3s(He) < E_{3s(H)
View Solution
The atomic numbers of elements A, B, D, E respectively are \(7, 17, 13, 11\). The element which forms basic oxide among them is
View Solution
Step 1: Identifying the Elements
The atomic numbers of the given elements correspond to:
- A (7): Nitrogen (N)
- B (17): Chlorine (Cl)
- D (13): Aluminum (Al)
- E (11): Sodium (Na)
Step 2: Determining the Nature of Their Oxides
- Nitrogen (\(N\)) forms acidic oxides like \(NO_2, N_2O_5\).
- Chlorine (\(Cl\)) forms acidic oxides like \(Cl_2O_7\).
- Aluminum (\(Al\)) forms an amphoteric oxide \(Al_2O_3\).
- Sodium (\(Na\)) forms a basic oxide \(Na_2O\), which dissolves in water to form \(NaOH\), a strong base.
Step 3: Conclusion
Since basic oxides are formed by metals, Sodium (Na) (element E) is the correct answer.
Thus, the correct answer is \( \mathbf{(1)} \) E. Quick Tip: Oxides of metals are generally basic, while oxides of nonmetals are acidic. Amphoteric oxides (e.g., \(Al_2O_3\)) show both acidic and basic behavior.
The electron gain enthalpy (in kJ/mol) of oxygen, sulphur, selenium respectively are
View Solution
Among the following, the molecule in which all atoms obey the octet rule is
View Solution
Identify the number of molecules having permanent dipole moment from the following:
\[ CCl_4, NF_3, H_2S, HBr, SF_4, SiF_4, XeF_4, BeCl_2, SnCl_2, BrF_5, SO_2 \]
View Solution
What is the correct equation that relates kinetic energy (\(E_{ke}\)) and pressure (\(P\)) of one mole of an ideal gas?
- (V = Volume)
View Solution
A hydrocarbon has 85.7% of carbon by weight. 56 g (2 moles) of hydrocarbon was completely burnt in oxygen and obtained \( CO_2 \) and \( H_2O \). What is the weight (in g) of \( CO_2 \) formed?
- (C = 12 u, H = 1 u, O = 16 u)
View Solution
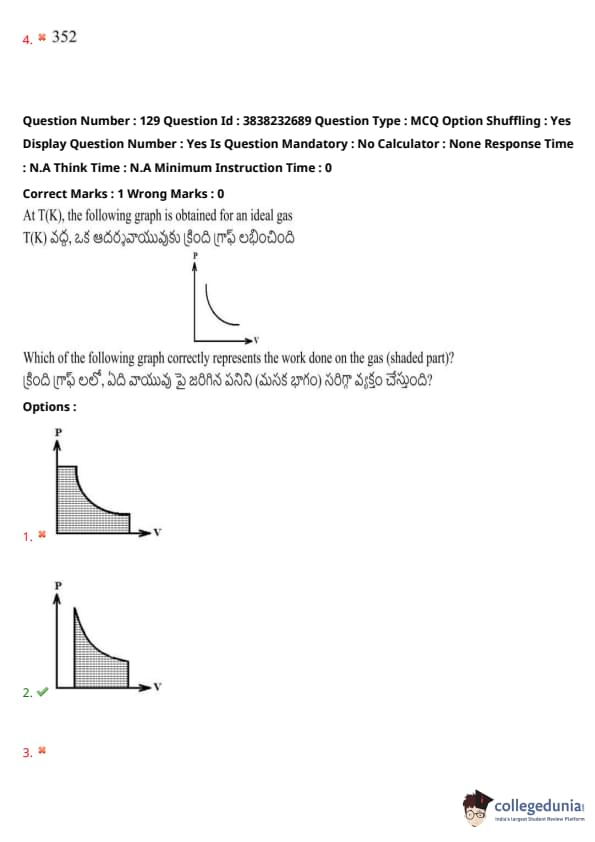
At T(K), the following graph is obtained for an ideal gas.
- Which of the following graphs correctly represents the work done on the gas (shaded part)?
View Solution
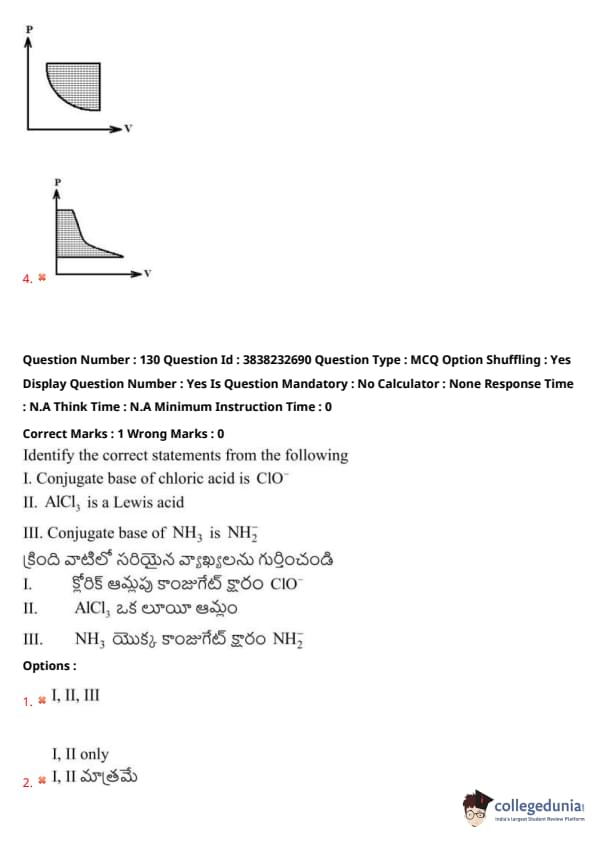
Identify the correct statements from the following:
I. Conjugate base of chloric acid is \( ClO^- \)
II. \( AlCl_3 \) is a Lewis acid
III. Conjugate base of \( NH_3 \) is \( NH_2^- \)
View Solution
Identify the correct statements from the following:
I. \( H_2O_2 \) oxidizes PbS to \( PbSO_4 \)
II. \( H_2O_2 \) is used in the industrial preparation of sodium perborate
III. \( H_2O_2 \) is insoluble in water
View Solution
The anions present in baking soda, caustic soda, and washing soda respectively are
View Solution
The products formed in the reaction of \( BeCl_2 \) with \( LiAlH_4 \) are
View Solution
Identify the reaction in which hydrogen gas is not liberated
View Solution
Assertion (A): Silicones are used in surgical and cosmetic plants
Reason (R): Silicones have high thermal stability
View Solution
The minimum concentration (ppm) of dissolved oxygen in water that is required for the growth of fish is
View Solution
Observe the following formula
The groups/atoms in the plane of the paper are
View Solution
IUPAC name of neohexyl alcohol is
View Solution
From the following, identify the set that contains all meta directing groups:
View Solution
Two statements are given below
Statement I: The resonance structure with more number of covalent bonds is less stable.
Statement II: The position of nuclei does not change in resonance structures.
View Solution
A crystal is formed by X (cations) and Y (anions). Atoms of Y form ccp and atoms of X occupy half of octahedral voids and half of tetrahedral voids. What is the molecular formula of the crystal?
View Solution
Two statements are given below
Statement I: Liquid A and liquid B form a non-ideal solution with positive deviation.
\hspace{0.5cm The interactions between A and B are weaker than A–A and B–B interactions.
Statement II: For an ideal solution, \(\Delta_{mix} H = 2 kJ mol^{-1} \); \(\Delta_{mix} V = 0\).
View Solution
At 300 K, aqueous KCl and aqueous K\(_2\)SO\(_4\) solutions were electrolyzed separately using Pt electrodes. The gases liberated at cathodes in these two electrolytic processes are respectively:
View Solution
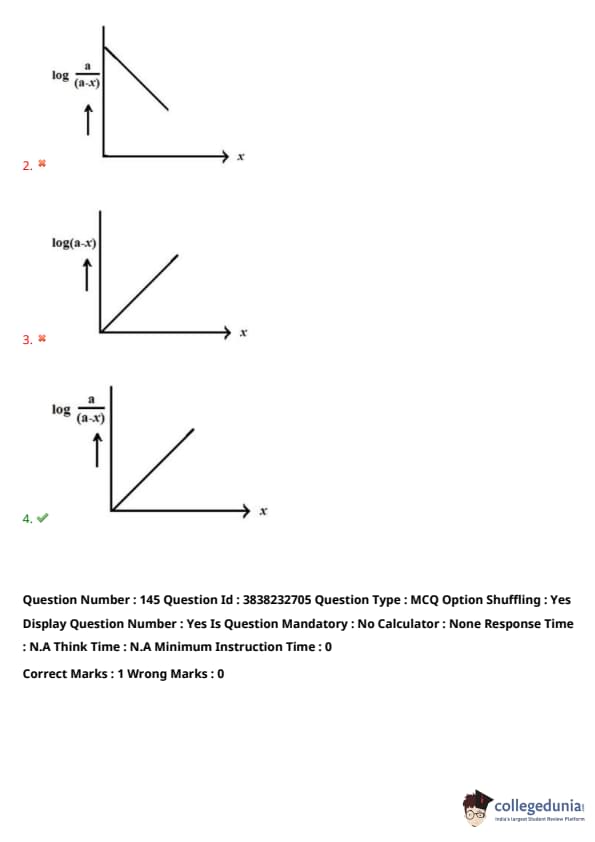
Identify the correct graph for a first-order reaction (A → P)
Given:
- \( x \)-axis = time (\( t \))
- \( a \) = Initial concentration of A
- \( (a-x) \) = Concentration of A at time \( t \)
View Solution
A few sols are given below:
As_2S_3 sol, starch sol, Al_2O_3 \cdot xH_2O sol, TiO_2 sol, gold sol, congo red sol, blood, methylene blue sol, CdS sol
The number of positively charged sols in the above list is:
View Solution
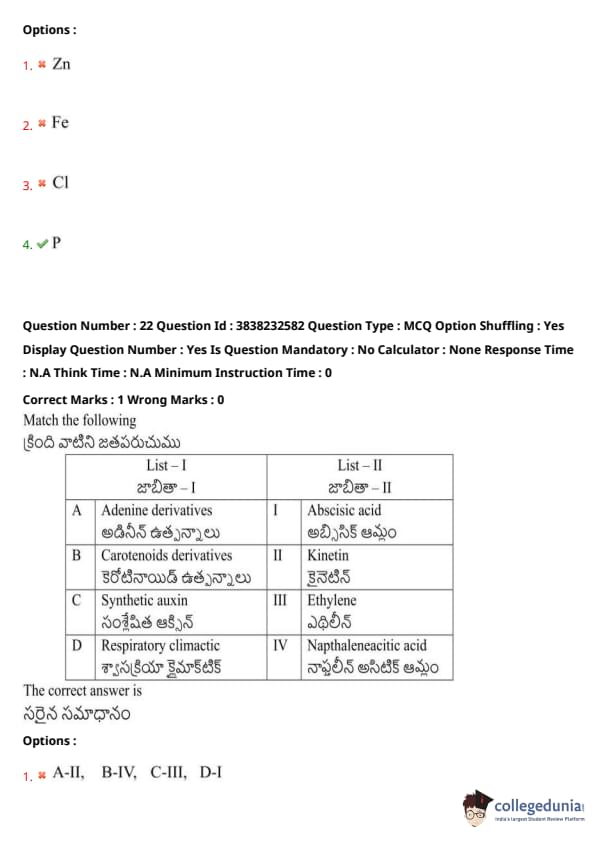
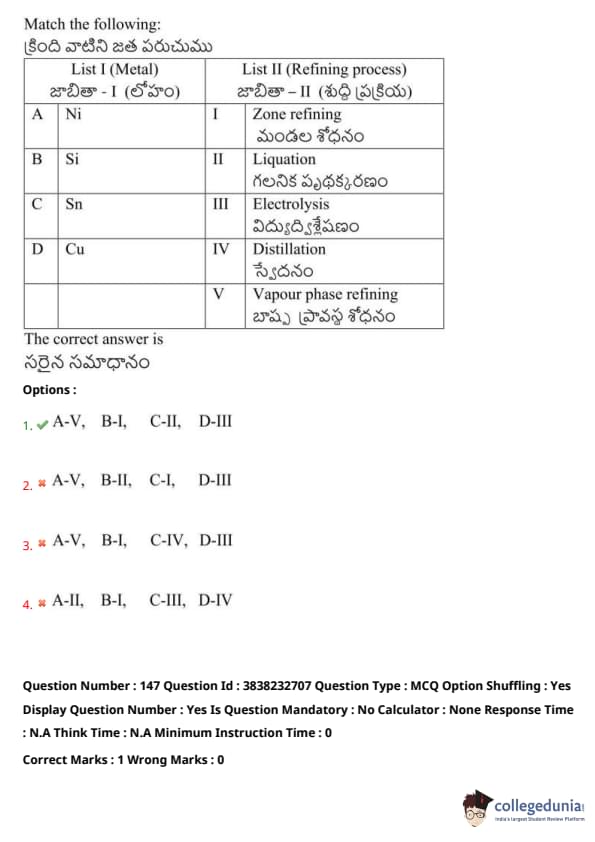
Match the following:
View Solution
Identify the correct statements from the following:
View Solution
The iodine oxide which is used in the estimation of carbon monoxide is:
View Solution
Which of the following oxoacids of phosphorus has two P-H bonds?
View Solution
The metal having the highest melting point among lanthanides is
View Solution
Which of the following is correct?
View Solution
Match the following:
View Solution
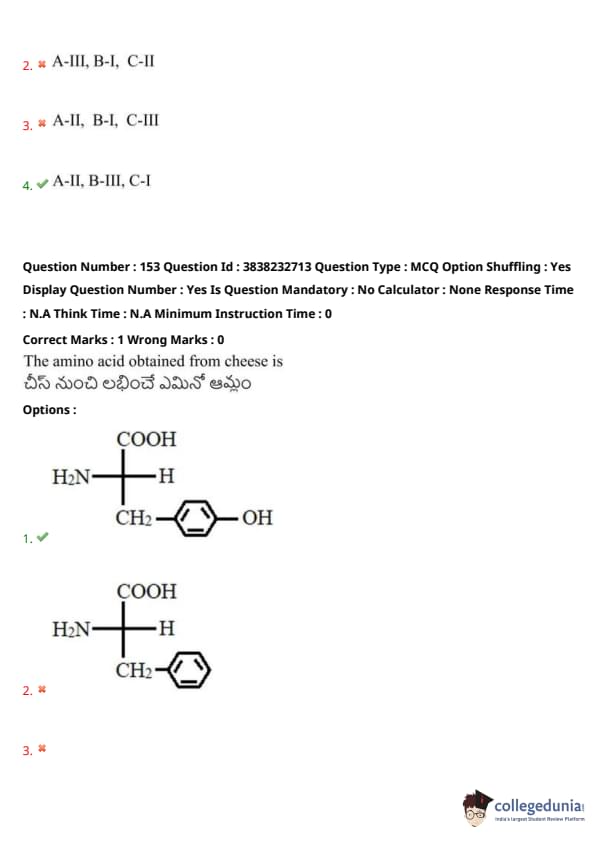
The amino acid obtained from cheese is
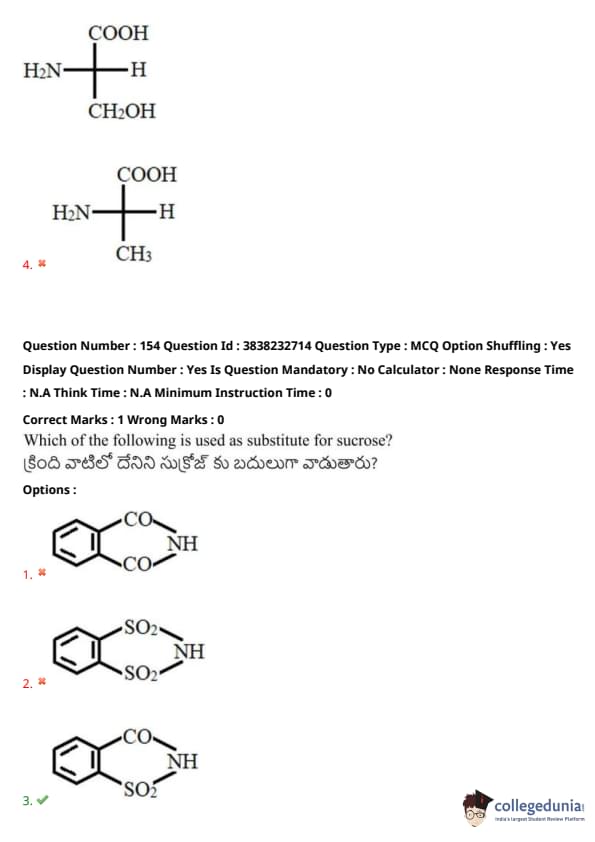
Which of the following is used as a substitute for sucrose?
An alkene \( X \) (\( C_5H_{10} \)) on reaction with \( HBr \) gave \( Y \) (\( C_5H_{11}Br \)). \( Y \) undergoes hydrolysis via an \( S_N1 \) mechanism. What is \( X \)?
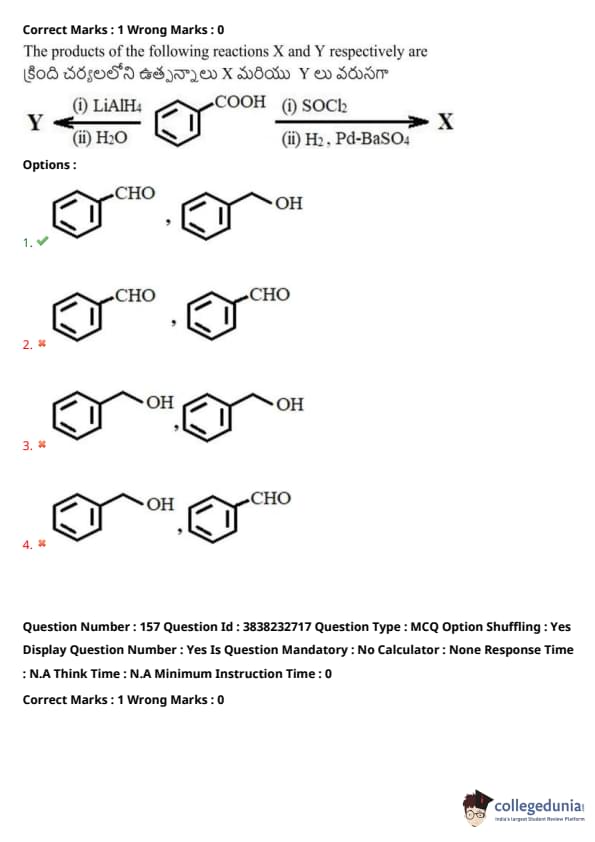
The products of the following reactions \( X \) and \( Y \) respectively are
Reaction:
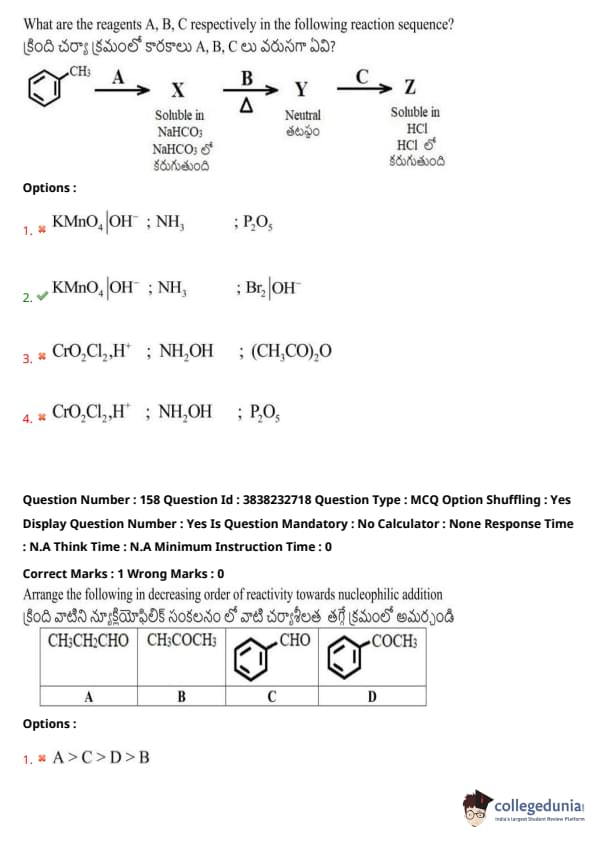
What are the reagents A, B, C respectively in the following reaction sequence?
Reaction Sequence:
View Solution
Arrange the following in decreasing order of reactivity towards nucleophilic addition.
Given compounds:
View Solution
Which of the following sequence of reagents converts benzoic acid to benzaldehyde?
View Solution
An organic compound \( C_7H_5N \) on reduction with reagent X gave Y. Reaction of Y with p-toluene sulphonyl chloride gave Z which is insoluble in alkali. X and Y respectively are:









































































































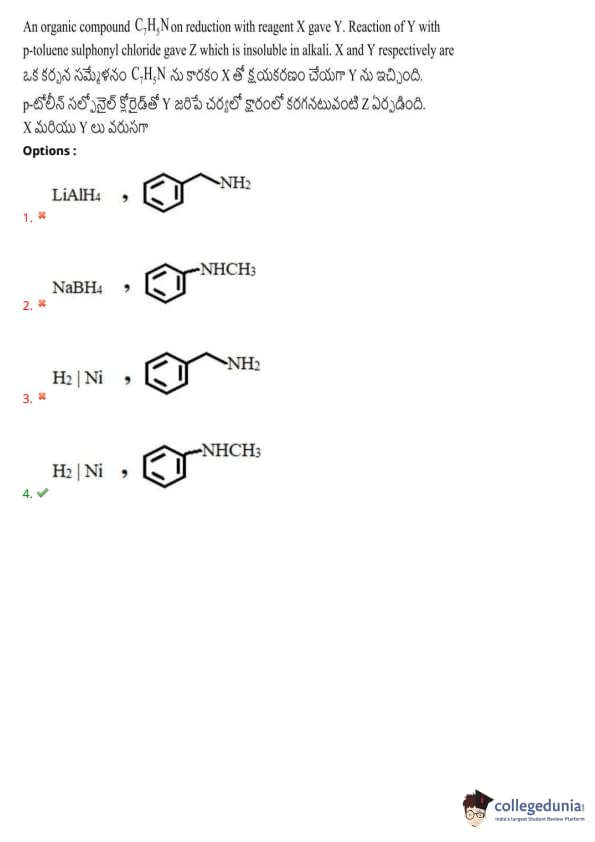
Also Check:
TS EAMCET Previous Year Question Papers
| TS EAMCET 2023 Question Paper | TS EAMCET 2022 Question Paper |
| TS EAMCET 2021 Question Paper | TS EAMCET 2020 Question Paper |
| TS EAMCET 2019 Question Paper | TS EAMCET 2018 Question Paper |
Also Check:



Comments