TS EAMCET 2024 Question Paper May 7 Shift 2 with Answer Key PDF is available here for download. JNTU, Hyderabad on behalf of TSCHE conducted TS EAMCET on May 7 from 3 PM to 6 PM. TS EAMCET 2024 Question Paper consists of 160 questions carrying 1 mark each. TS EAMCET 2024 Question Paper May 7 Shift 2 PDF for BiPC includes four subjects, Physics, Chemistry and Biology with Botany & Zoology. Each subject includes 40 questions.
TS EAMCET 2024 Question Paper with Answer Key May 7 Shift 2 PDF
| TS EAMCET 2024 Question Paper with Answer Key | Check Solution |
The question asks for an example of RNA-containing viruses.
View Solution
Asexual spores are generally not found in:
View Solution
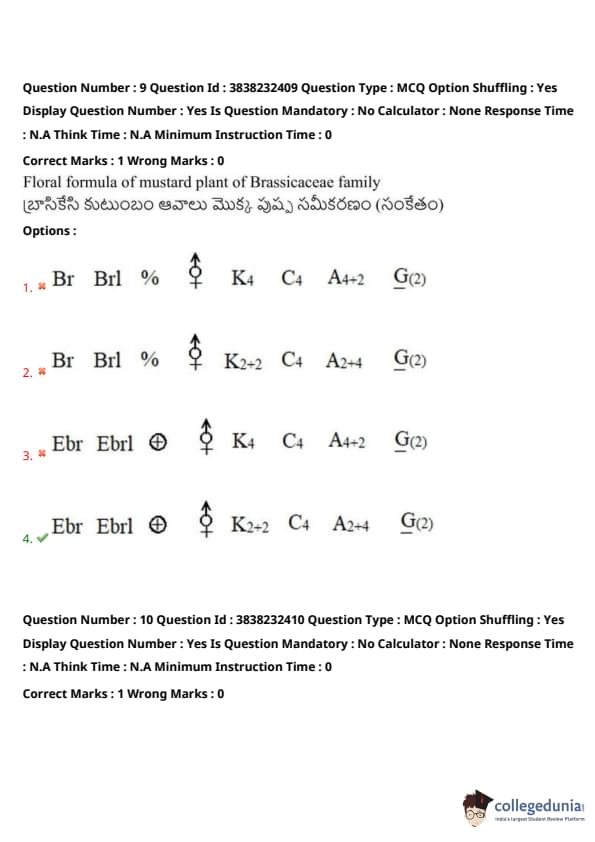
Match the following:
The correct answer is
View Solution
Sexual reproduction in plants was discovered by:
View Solution
Identify true sentences regarding leaf modifications in Nepenthes:
A. Petiole upper part is modified into tendril.
B. Petiole lower part is modified into phyllode.
C. Lamina is modified into pitcher.
D. Petiole lower part is modified into lid of pitcher.
View Solution
Male flowers, female flowers and sterile gall flowers are present in:
View Solution
Identify incorrect match:
A. Asparagus - Cladode - Balancing roots
B. Bougainvillea - Thorn - Runner
C. Dioscorea - Bulbil - Vegetative reproduction
D. Casuarina - Phylloclade - Xerophyte
View Solution
Which of the following statement is correct?
View Solution
Floral formula of mustard plant of Brassicaceae family is:
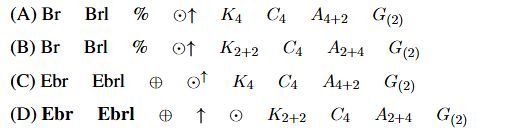
View Solution
Characters of sunflower ovule.
I. Micropyle lie close to funicle
II. Inverted ovule
III. Ovule curvature is 180°
IV. Curved embryo sac
View Solution
Which of the following is not a function of cytoskeleton?
View Solution
‘A’ diploid chromosome number is 4. ‘B’ haploid chromosome number is twice to that of A. ‘C’ diploid chromosome number is thrice to that of A. A, B and C respectively are
View Solution
Identify the wrong statement of the following:
View Solution
Match the following:
The correct answer is
View Solution
Primary endosperm nucleus is formed by the fusion of
View Solution
Identify the correct matching:
View Solution
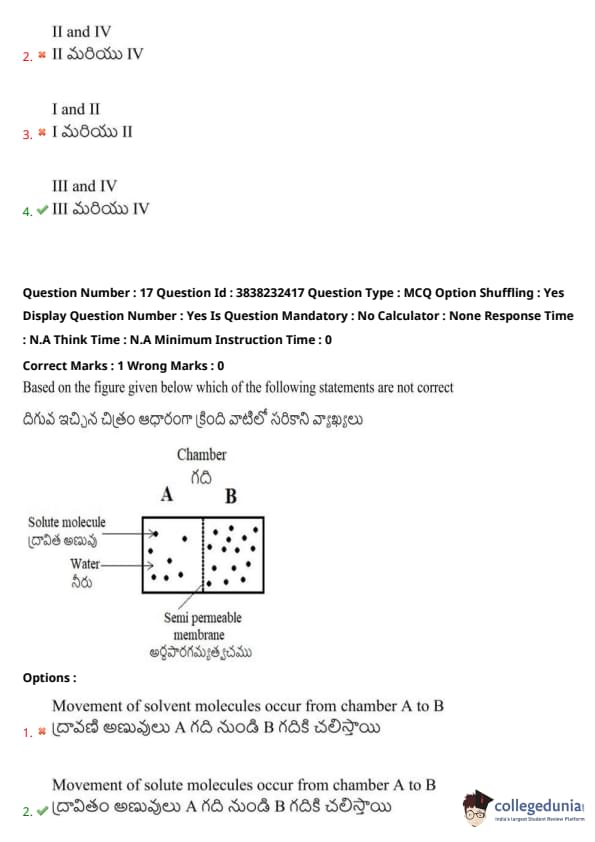
Based on the figure given below, which of the following statements are not correct?
View Solution
Match the following:
The correct answer is
View Solution
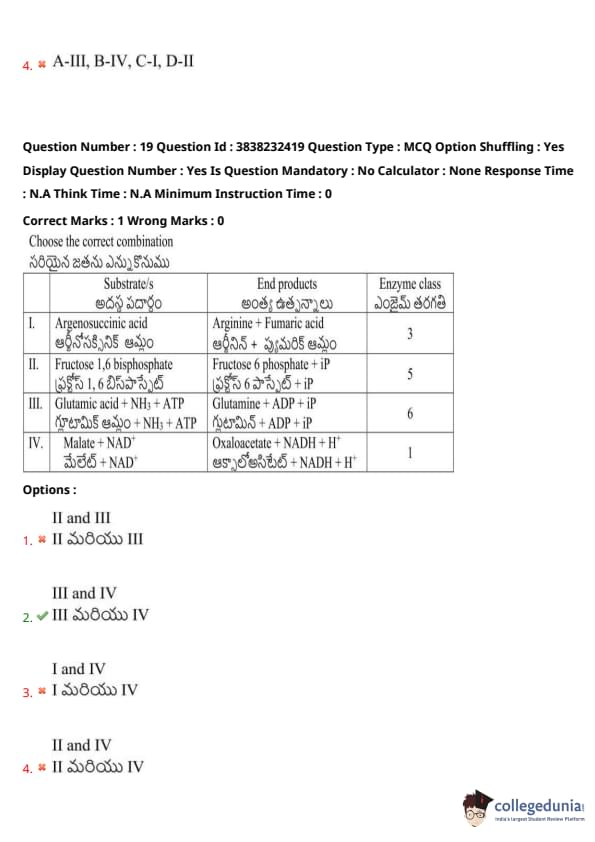
Choose the correct combination
View Solution
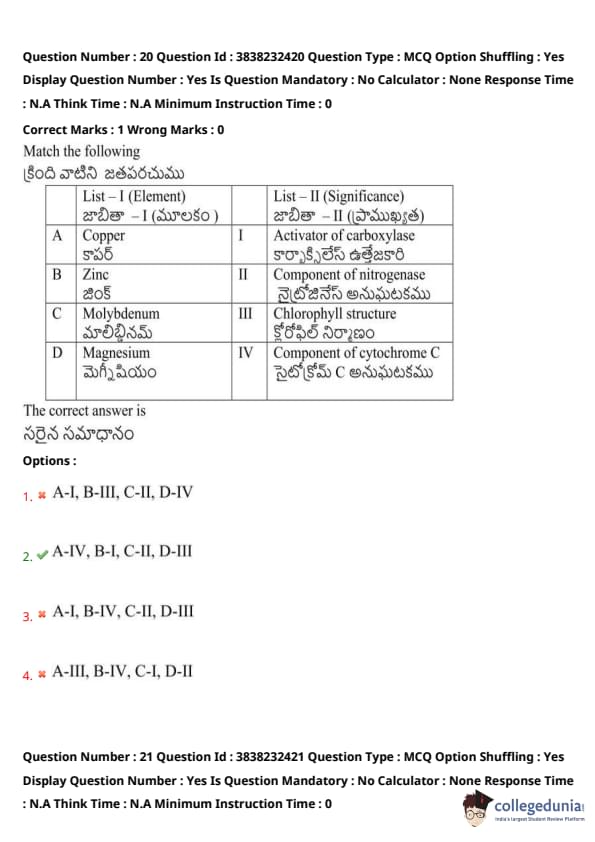
Match the following:
View Solution
Assertion (A): In Arachis, fruits are geocarpic.
Reason (R): Seeds are non-endospermic in Arachis.
View Solution
Identify the wrong match.
I. Acidy amino acid Valine
II Neutral amino acid Tryptophan
III. Aromatic amino acid Tyrosine
IV. Basic amino acid Lysine
View Solution
Identify correct statements
(1) In C\(_4\) plants PEP carboxylase is present in mesophyll cells.
(2) In C\(_4\) plants mesophyll cells lack RuBisCO enzyme.
View Solution
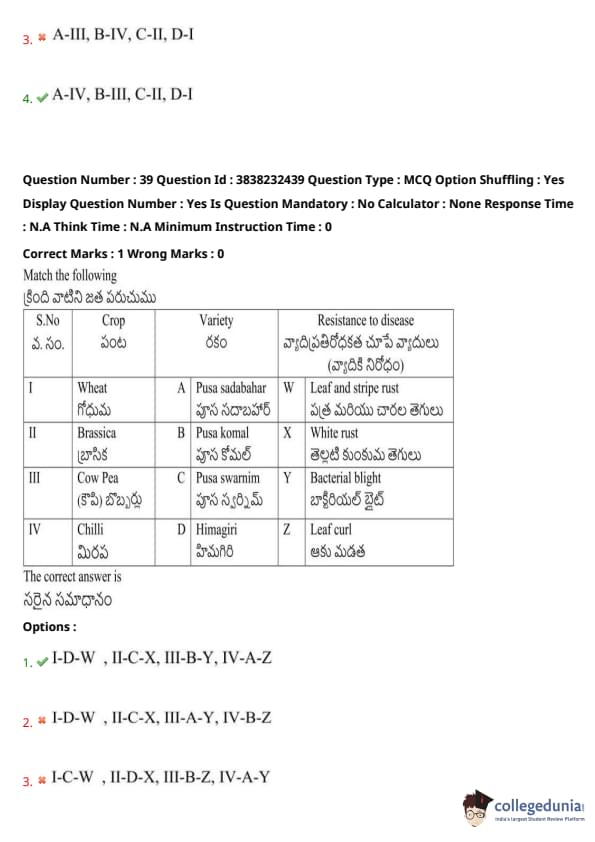
Match the following
The correct answer is:
(1) A-II, B-III, C-IV, D-I
View Solution
Identify the enzyme of the following reaction: Phosphoglyceric acid + ATP \xrightarrow{Enzyme} Bisphosphoglyceric acid + ADP
View Solution
Observe the diagrammatic representation of ATP synthesis in oxysome of mitochondria. Identify A, B and C respectively.
View Solution
Find out A, B, C, and D products of anaerobic respiration pathway cited below
View Solution
Assertion (A): Abscisic acid is called stress hormone.
Reason (R): Abscisic acid increases the tolerance of plants to various kinds of stresses.
View Solution
Mad Cow disease causing prion, may reach man through beef and cause
View Solution
Match the following
The correct answer is
View Solution
The separated DNA fragments can be visualised after staining with 'X' and exposure to 'Y'. The 'X' and 'Y' are
View Solution
Assertion (A): \( \sigma \) (Sigma) and \( \rho \) (Rho) factors are essential for transcription in bacteria.
Reason (R): \( \sigma \) (sigma) factor helps in initiation and \( \rho \) (Rho) factor helps in termination of transcription.
View Solution
Dual functions of codon AUG
View Solution
Identify wrong matching
I. Roundup ready = Soyabean = Herbicide tolerant
II. Transgenic Plant = Papaya = Pseudomonas resistant
III. Taipei variety = Rice = rich in Vitamin B
IV. Flavr savr = Tomato = Bruise resistant
View Solution
Assertion (A): Protoxin of Bacillus thuringensis become active in gut of insects.
Reason (R): Alkaline pH of intestine gut solubilizes the crystals of toxin to make it active.
View Solution
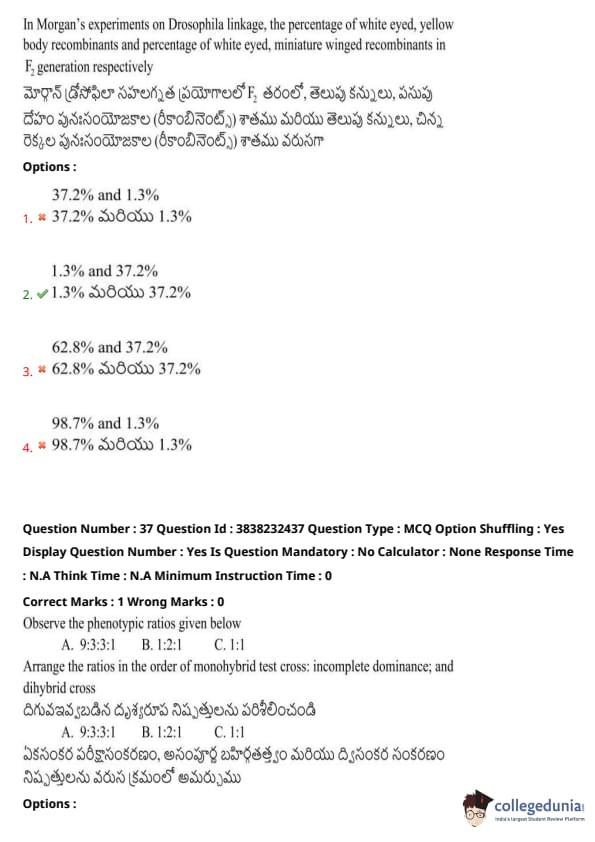
In Morgan’s experiments on Drosophila linkage, the percentage of white-eyed, yellow body recombinants and the percentage of white-eyed, miniature winged recombinants in F\textsubscript{2} generation respectively:
View Solution
Arrange the ratios in the order of monohybrid test cross: incomplete dominance; and dihybrid cross.
View Solution
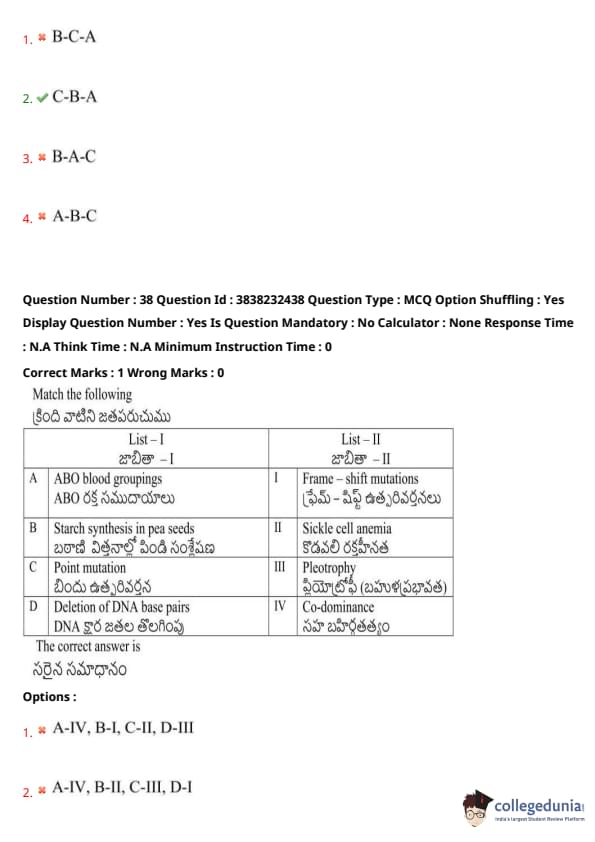
Match the following
The correct answer is
View Solution
Match the following:
View Solution
Cyclosporin A is produced by:
View Solution
Study the following and pick up the correct statements:
[(I)] All the threatened species are listed in red data book
[(II)] National parks, Sanctuaries, Biosphere Reserves etc. are some of the methods of ex-situ conservation
[(III)] An area which is set aside, minimally disturbed for conservation of the resources of an area is called Biosphere Reserve
[(IV)] \(\beta\)-diversity is measured by counting the number of taxa within a particular area
View Solution
Study of cell as a structural and functional unit of living organisms is called:
View Solution
Assertion (A): Pancreas is a merocrine gland.
Reason (R): The apical parts of the pancreatic cells are pinched off along with the secretory product.
View Solution
Match the following
The correct answer is
View Solution
Parazoans exhibit:
View Solution
Study the following and pick up the correct combinations:
View Solution
Botryoidal tissue in body cavity is characteristic of:
View Solution
Match the following:
The correct answer is
View Solution
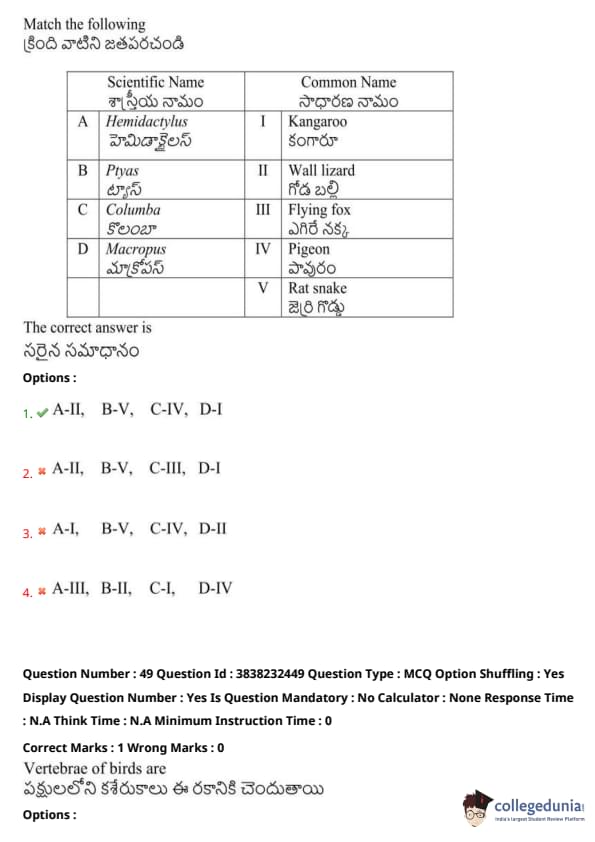
Vertebrae of birds are
View Solution
Pseudopodia in Euglpha are
View Solution
Study the following and pick up the correct statements:
(I) Due to Sacculina, its host becomes sterile
(II) Plasmodium frequently changes its surface antigens
(III) Fasciola causes hypertrophy of RBC in sheep
(IV) Malaria is spread by female Culex
View Solution
Assertion (A) : Eggs of Ascaris are described as mamillated eggs.
Reason (R) : Each egg is surrounded by a protein coat with rippled surface.
View Solution
Marijuana, hashish, charas and ganja are described as
View Solution
Identify the hyperparasite
View Solution
In cockroach, depression of wings is due to contraction of
View Solution
In cockroach expiration takes place through
View Solution
Statement I: Spiral cuticular thickenings of trachea in cockroach are called taenidia.
Statement II: In cockroach, inspiration is a passive process.
View Solution
The oriented locomotor movements of an organism towards or away from light is known as
View Solution
Statement I:Natural aging of a lake by enrichment of nutrients is known as eutrophication.
Statement II:Increase of concentration of pollutant at successive trophic levels in aquatic food chain is known as biomagnification.
View Solution
Match the following
The correct answer is
View Solution
These enzymes function actively in acidic medium.
View Solution
Assertion(A) :The reaction between \( CO_2 \) and \( H_2O \) in the RBC is much faster than in blood plasma.
Reason(R) :Red blood cells contain a very high concentration of carbonic anhydrase
View Solution
Statement I:Angina pectoris is a warning signal of deprivation of blood supply to the heart muscles.
Statement II:Coronary artery disease is also called atherosclerotic heart disease.
View Solution
During glomerular filtration, net filtration pressure that causes filtration of blood in glomerular capillaries of Bowman's capsule is
View Solution
The thick filaments in a sarcomere of a myofibril are held together by a thin fibrous membrane called
View Solution
Study the following and pick up the incorrect statements:
I.The axolemma has more \( K^+ \) leakage channels than \( Na^+ \) leakage channels.
II.Ligand gated channels open or close in response to electrical stimuli.
III.Voltage gated channels open in response to a change in membrane potential.
IV.Speed of conduction of nerve impulse is more in non myelinated nerve fibre than the myelinated nerve fibre.
View Solution
Study the following and pick up the correct combinations:
View Solution
The secretions of this gland play major role in differentiation of T- lymphocytes.
View Solution
Match the following:
The correct answer is
View Solution
These glands of the female reproductive system of human beings are homologous to the prostate gland of male:
View Solution
Pick up the incorrect pair:
View Solution
If karyotype of a Drosophila is AA-XXXY, its sexual phenotype is
View Solution
It is an example for monosomy
View Solution
Vision of the children born to a colour blind father and normal homozygous mother is
View Solution
Seymouria is a transitional form between
View Solution
Statement I:Disruptive selection operates when homogeneous environment changes into a heterogeneous type.
Statement II: Change the frequency of a gene that occurs by chance and not by selection in small populations is called directional selection
View Solution
Existence of deleterious genes in a population is called:
View Solution
Assertion (A): Influenza vaccine is an inactivated whole agent vaccine.
Reason (R): It contains killed microbes.
View Solution
Tall T-wave in an ECG indicates ECG
View Solution
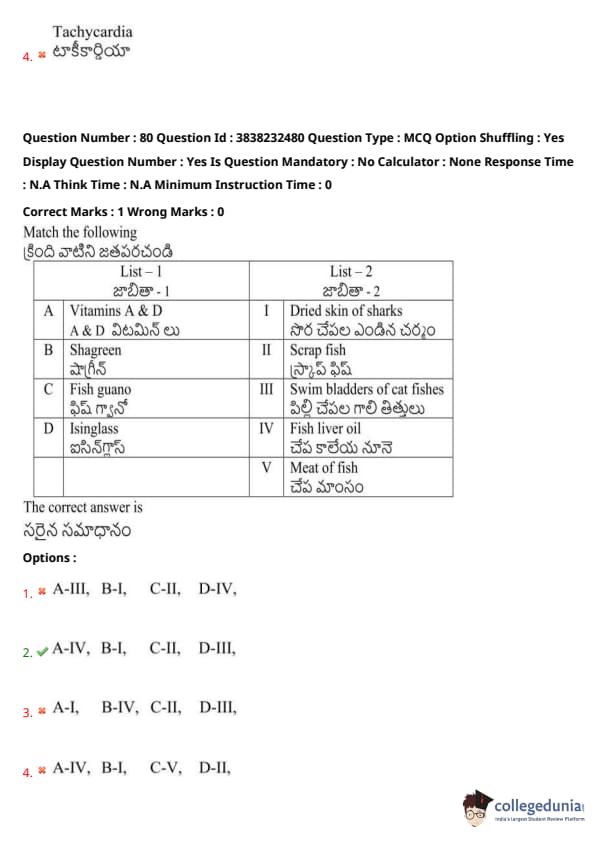
Match the following
The correct answer is
(1) A-III, B-I, C-II, D-IV
View Solution
The theory currently accepted as proper framework for explaining microscopic phenomena is
View Solution
The displacement of a particle in wave motion is given by:
y = a \sin (\beta x + \gamma t) where \( x \) and \( t \) represent displacement and time, respectively. Then, the dimensional formula for
\frac{\beta{\gamma is:
View Solution
A body thrown vertically upwards with certain velocity from the ground reaches a maximum height \( H \). The ratio of the times at which the body is at a height of \( \frac{H}{2} \) is:
View Solution
The maximum range of a projectile is 80 m. If the projectile is projected with the same speed at an angle of \( \frac{\pi}{12} \) with the horizontal, then the range of the projectile is:
View Solution
A force of 20 N acts on a body at rest for a time of 2 s and then a force of 60 N acts for a time of 1.5 s in the opposite direction. If the final velocity of the body is 10 m/s in the direction of the 60 N force, then the mass of the body is:
View Solution
A body of mass 4 kg is falling freely from rest from a height of 30 m from the ground. If the velocity of the body when it is at a height of 10 m from the ground is 10 m/s\(^1\), then the loss of energy due to air resistance on the body is:
View Solution
A ball of mass 200 g moving with certain velocity collides with another ball of mass 600 g at rest.
If the coefficient of restitution is 0.6, the ratio of the velocity of 600 g ball after collision
and the velocity of 200 g ball before collision is:
View Solution
A solid sphere is rolling on a horizontal surface without slipping. The ratio of the translational and rotational kinetic energies of the sphere is
View Solution
Two particles each of mass \(m\) are separated by a distance \(d\). If the mass of one of the particles is doubled without changing the distance between the two particles, then the shift in the position of the center of mass is
View Solution
The time period of a simple pendulum on the surface of the earth is \( T \). The time period of the same pendulum at a height of 1280 km from the surface of the earth is
View Solution
The escape speed of a body from the surface of the earth is 11.2 km/s.
The escape speed of a body from the surface of a planet whose mass is 8 times that of the earth
and mean density same as that of the earth is:
View Solution
If the length of a cylinder made with a material of Poisson’s ratio 0.4 is increased by 5%, then the decrease in its diameter is:
View Solution
A 31.4 kg girl wearing high heel shoes balances on a single heel. The heel is circular with a diameter of 2 cm. The pressure exerted by the heel on the horizontal floor is (Acceleration due to gravity = 10 m/s\(^2\)):
View Solution
Two soap bubbles of volumes \( 27V \) and \( 64V \) coalesce under isothermal conditions.
The volume of the bigger bubble formed is:
View Solution
Consider an isolated system of two concentric spherical black bodies.
The inner sphere of radius \( R \) is at temperature \( T \),
and the outer sphere of radius \( 4R \) is at temperature \( 2T \).
The rate of absorption of radiant energy by the outer sphere is:
View Solution
Three identical rods are joined as shown in the figure.
The left and right ends are kept at \( 0^\circ C \) and \( 90^\circ C \) as shown in the figure.
The temperature \( \theta \) at the junction of the rods is:
View Solution
The gas that gives the highest fractional conversion of heat to work in an isobaric process is:
View Solution
If the average speed of molecules of an ideal gas in a container is doubled
and the volume of the container is halved, then the increase in the pressure of the gas is:
View Solution
A and B are two points of a string, in which a standing wave of wavelength \( \lambda \) is set up.
If the distance between the points A and B is \( \frac{3\lambda}{4} \), then the phase difference between A and B is:
View Solution
Consider the wave represented by the equation \( y = (0.02) \sin \left( \pi x + 8\pi t \right) \), where all quantities are in SI units. The wavelength and speed of this wave respectively are \( y = (0.02) \sin \left( \pi x + 8\pi t \right) \).
View Solution
A convex lens of focal length 20 cm is immersed in a liquid of refractive index 1.3.
If the refractive index of the material of the lens is 1.5, then the focal length of the lens
when immersed in the liquid is:
View Solution
When a light ray incident on an equilateral prism, the angle of minimum deviation is found to be half of the angle of prism. The refractive index of the material of the prism is:
View Solution
In Young's double slit experiment, the wavelength of the monochromatic light used is \( \lambda \), the distance between the slits is \( 5\lambda \) and the distance of the screen from the plane of the slits is 100 cm. If the maximum intensity on the screen is \( I_0 \), then the intensity at a point on the screen which is at 5 cm from the central maximum is:
View Solution
Sixteen point charges each of charge \( q \) are placed on the circumference of a circle of radius \( r \) with equal angular spacing. If one of the charges is removed, then the net electric field at the centre of the circle is \( \varepsilon_0 \) (where \( \varepsilon_0 \) is the permittivity of free space). What is the net electric field at the centre?
View Solution
The radii of two conducting spheres A and B of each charge +90 µC are 8 cm and 10 cm respectively. When the two spheres are connected by a conducting wire, then the charge flowing from sphere A to sphere B is:
View Solution
The relation between the charge \( Q \) (in coulombs) passing through a resistor of resistance 200 \(\Omega\) and the time of flow of charge \( t \) (in seconds) is \( Q = 3t - 4t^2 \). The total heat produced in the resistor up to the time when instantaneous current becomes zero is:
View Solution
A cell of emf 2 V is connected to an external resistor. If the current through the resistor is 200 mA and the terminal voltage of the cell is 87.5% of the emf of the cell, then the internal resistance of the cell is:
View Solution
A galvanometer of resistance 99.9 \( \Omega \) gives a full scale deflection when 5 mA current is passed through it. The resistance to be connected to the galvanometer such that it can be converted into an ammeter of range 0-5 A is:
View Solution
A current carrying wire is first bent in the form of a circular loop and then bent in the form of a square loop. The ratio of the magnetic fields induced at the centres of the loops in the two cases is:
View Solution
A paramagnetic substance in the form of a cube of side 3 cm has a magnetic moment of \(243 \times 10^{-6}\) Am\(^2\), when a magnetic field of intensity \(150 \times 10^{3}\) Am\(^{-1}\) is applied. The susceptibility of the substance is:
View Solution
A coil of 100 turns and 0.10 m\(^2\) area, making two rotations per second is placed in a 0.01 T uniform magnetic field perpendicular to its axis of rotation. The maximum voltage generated in the coil is:
View Solution
Resonance phenomenon is exhibited by a circuit only if:
View Solution
The physical quantity which has the same value for green light, \(\gamma\)-radiation, and X-rays is:
View Solution
A photoelectron emitted when a light of wavelength 2480 \(\mathring{A}\) falls on a metal, enters a uniform magnetic field of \( \frac{1}{4} \times 10^{-5} \) T perpendicular to it and moves in a circular path of maximum radius 1 m. The work function of the metal is nearly:
View Solution
If the total energy of the electron in the ground state of hydrogen atom is \(-13.6\) eV, then the potential and kinetic energies of an electron in this state respectively are:
View Solution
If a substance decays from 32 g to 1 g in 25 days, then its half-life is:
View Solution
Reactor used to produce fissile material is:
View Solution
The energy gap between conduction and valence bands of silicon is:
View Solution
The current gain of a transistor in common emitter configuration is 45. If the resistances in collector and base sides of the circuit are 4.5 kΩ and 900 Ω, the voltage gain of the amplifier is:
View Solution
The height of a transmitting antenna is 320 m and the height of a receiving antenna is 20 m. The maximum distance between them for satisfactory communication in LOS mode is:
View Solution
Which of the following gives proof of quantized electronic energy levels in hydrogen atom?
View Solution
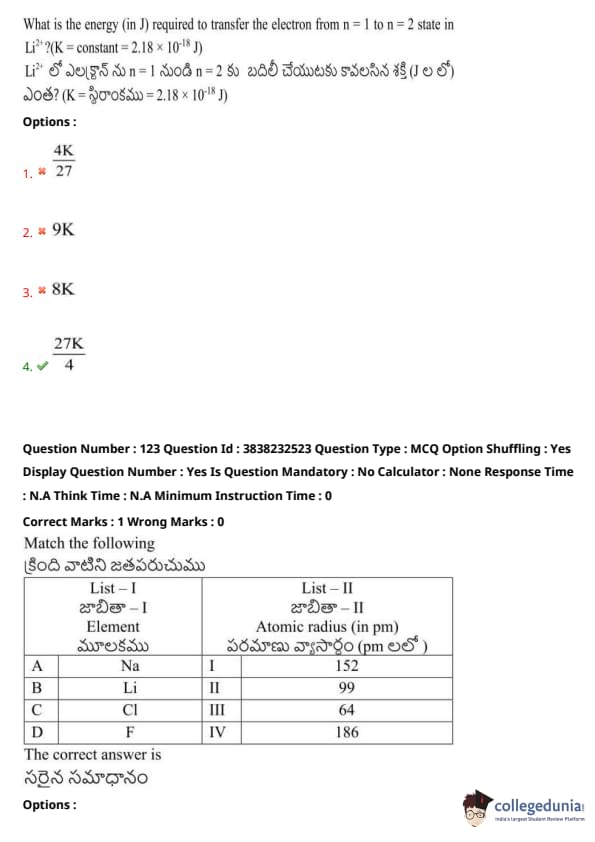
What is the energy (in J) required to transfer the electron from \( n = 1 \) to \( n = 2 \) state in \( Li^{2+} \)? (K = constant \( 2.18 \times 10^{-18} \) J)
View Solution
Match the following
The correct answer is
View Solution
The electronic configuration of four elements are given below:
The correct order of magnitude (without sign) of their electron gain enthalpies is:
View Solution
Identify the sets containing isostructural molecules from the following
I. \(SiF_4\), \(SF_4\)
II. \(IO_3^-\), \(XeO_3\)
III. \(BH_4^-\), \(NH_4^+\)
IV, \(PF_6^-\), \(SF_6\)
The correct option is
View Solution
Which one of the following order is correct regarding the covalent character of given molecules?
View Solution
At \( T(K) \), three moles of an ideal gas is present in a 10 L vessel. If the kinetic energy of an ideal gas is \( 3000 \) J mol\(^{-1}\), the approximate pressure of the gas (in atm) is:
View Solution
A hydrocarbon containing C and H has 92.3% of C. When 52 g of hydrocarbon is completely burnt in oxygen, \( x \) moles of water and \( y \) moles of CO\(_2\) were formed. The liberated water is sufficient to liberate one mole of H\(_2\) when reacted with sodium metal. What is the weight (in g) of O\(_2\) consumed?
View Solution
At \( T(K) \), a vessel contains \( V \) litres of an ideal gas. The vessel was partitioned into three equal parts. The volume (in L) and temperature (in K) in each part are respectively
View Solution
Identify the reaction for which \( K_p = K_c \):
View Solution
What is X in the following reaction? \[ CO(g) + 2H_2(g) \xrightarrow{X} CH_3OH(l) \]
View Solution
The number of products formed by thermal decomposition of lithium nitrate, sodium nitrate respectively are:
View Solution
Which of the following statements are not correct?
i) BeO has rock-salt structure
ii) BeSO_4 is readily soluble in water
iii) The maximum coordination number of beryllium is four
iv) Be(OH)_2 is basic in nature
View Solution
Observe the following compounds/ions: \[ H_3BO_3, \, [B(OH)_4]^-, \, BH_4^-, \, [BCI_3 \cdot NH_3], \, SiO_4^{2-} \]
The number of compounds/ions with tetrahedral shape is:
View Solution
Which of the following sets of oxides are correctly matched?
View Solution
Which radical is responsible for depletion of ozone in stratosphere?
View Solution
The number of benzenoid and non-benzenoid aromatic species present in the following list are respectively
Naphthalene, Toluene, Cycloheptatrienyl cation, Anthracene
View Solution
The IUPAC names of the compounds A and B respectively are
View Solution
0.1435 g of silver chloride was obtained from 0.0945 g of an organic compound by Carius method. The percentage of chlorine by weight in the compound is (molar mass of AgCl = 143.5 g mol\(^{-1}\))
View Solution
In the following reaction sequence X and Y respectively are
View Solution
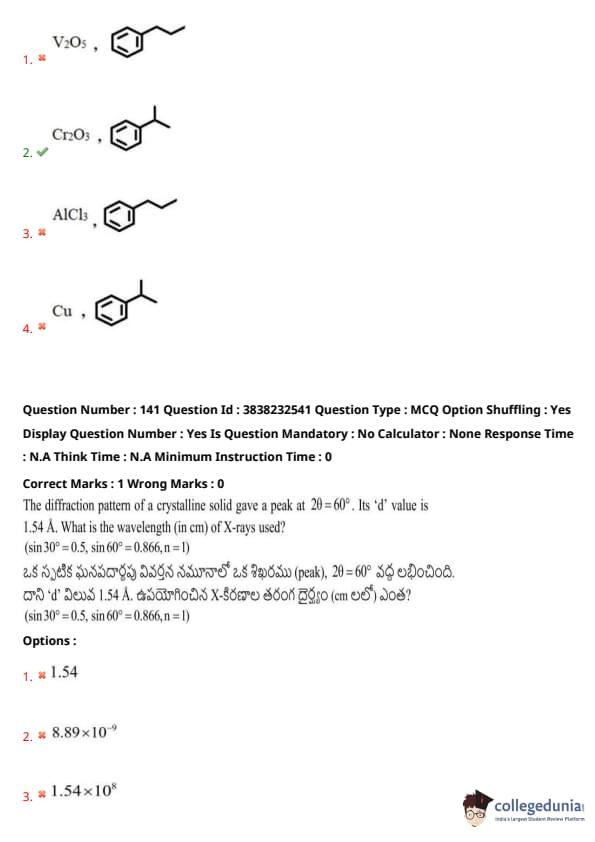
The diffraction pattern of a crystalline solid gave a peak at \( 2\theta = 60^\circ \). Its ‘d’ value is 1.54 Å. What is the wavelength (in cm) of X-rays used?
View Solution
The concentration of 1 L of CaCO\(_3\) solution is \(10^{-5}\) M. Its concentration in ppm is.
(Ca=40u; C=12u; O=16u)
View Solution
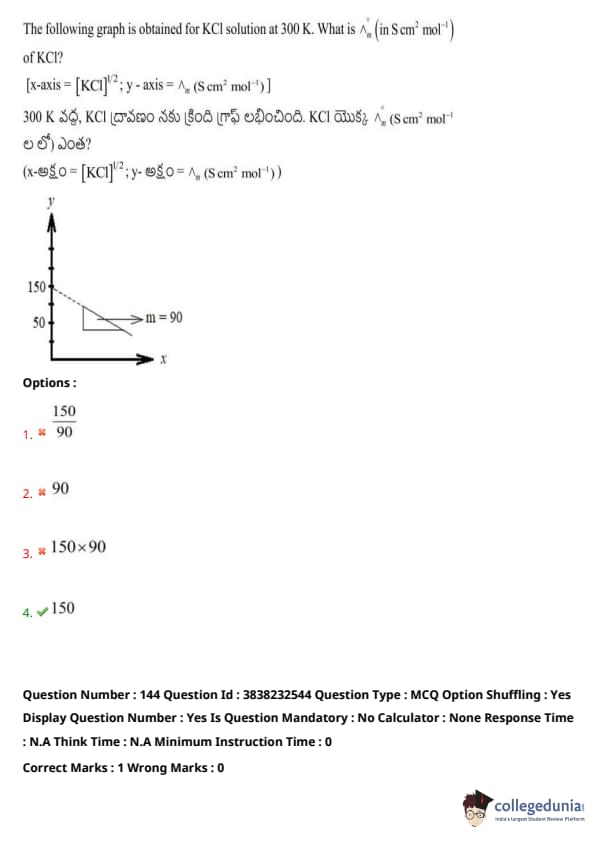
The following graph is obtained for KCl solution at 300 K. What is \( \Lambda_m^0 \) (in S cm² mol\(^{-1}\)) of KCl?
View Solution
Identify the correct statements from the following:
I. The order of reaction is determined from experiment only
II.The order of reaction can be zero , positive integer or a fraction
III.In a multistep reaction, the slow step determines the rate
View Solution
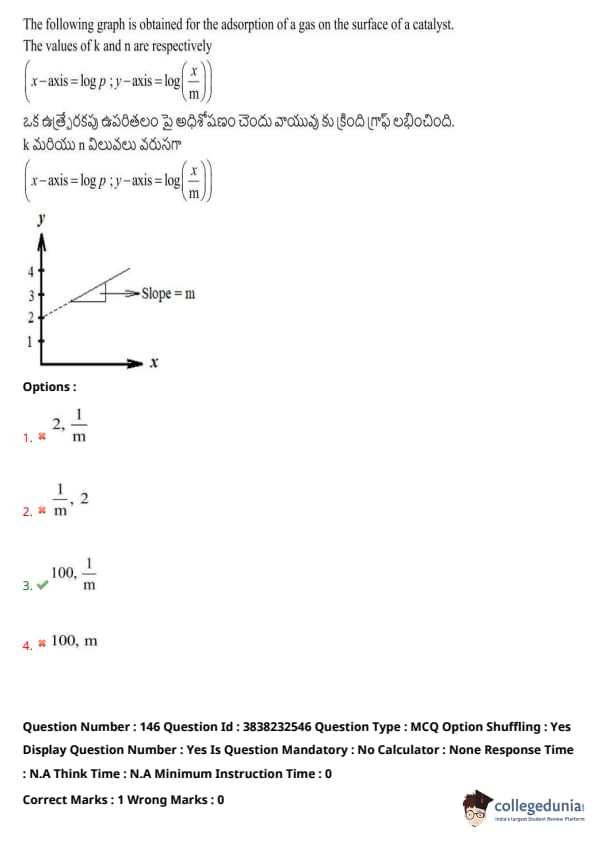
The following graph is obtained for the adsorption of a gas on the surface of a catalyst. The values of k and n are respectively
\left( x\text{-axis = \log p ; \, y\text{-axis = \log \left( \frac{x{m \right)
View Solution
In which of the following metal extraction, \( CO_2 \) is used?
View Solution
The oxide of nitrogen, 'X' is a blue solid and is acidic in nature. \( X \) is
View Solution
Observe the following reactions (not balanced)
\text{Cl_2 + \text{NaOH \rightarrow \text{NaCl + X + \text{H_2\text{O
\text{Cl_2 + \text{NaOH \rightarrow \text{NaCl + Y + \text{H_2\text{O
View Solution
Which of the following is not correct?
View Solution
Identify the oxidizing reactions of KMnO\(_4\) in acidic medium.
I.Liberation of iodine from KI
II.Conversion of \(Fe^{2+}\) to \(Fe^{3+}\)
III.Oxidation of nitrite to nitrate
IV.Oxidation of iodide to iodate
View Solution
The set of complex ions having the same number of unpaired electrons is
View Solution
A polymer contains 800 molecules of molar mass 1000, 100 molecules of molar mass 2000 and 100 molecules of molar mass 5000. What is its number average molecular weight (\(M_n\))?
View Solution
The vitamin that can be stored in the body and whose deficiency results in disease is:
View Solution
The drug which is obtained from opium poppy and its use are respectively:
View Solution
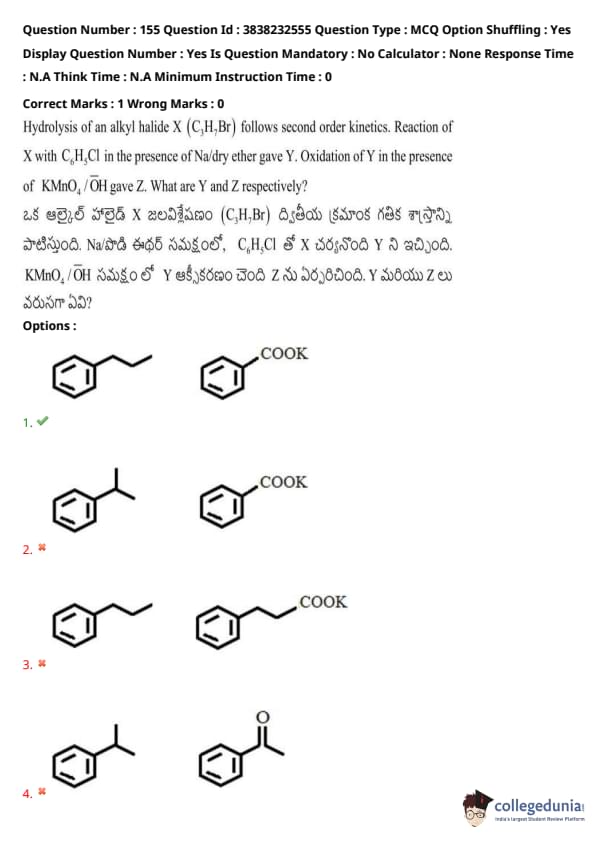
Hydrolysis of an alkyl halide \( X \) (\(C_6H_5Br\)) follows second order kinetics. Reaction of \( X \) with \( C_6H_5Cl \) in the presence of Na/dry ether gave \( Y \). Oxidation of \( Y \) in the presence of \( KMnO_4 / OH^- \) gave \( Z \). What are \( Y \) and \( Z \) respectively?
View Solution
A carbonyl compound \( X(C_3H_6O) \) on oxidation gave carboxylic acid \( Y(C_3H_6O_2) \). The oxime of \( X \) is:
View Solution
Two statements are given below:
Statement I: The boiling points of alcohols increase with increase of branching in carbon chain.
Statement II: The solubility of alcohol decreases with increase in size of alkyl group.
View Solution
Match the following carboxylic acids with their pKa values.
View Solution
The major product of the following reaction is
View Solution
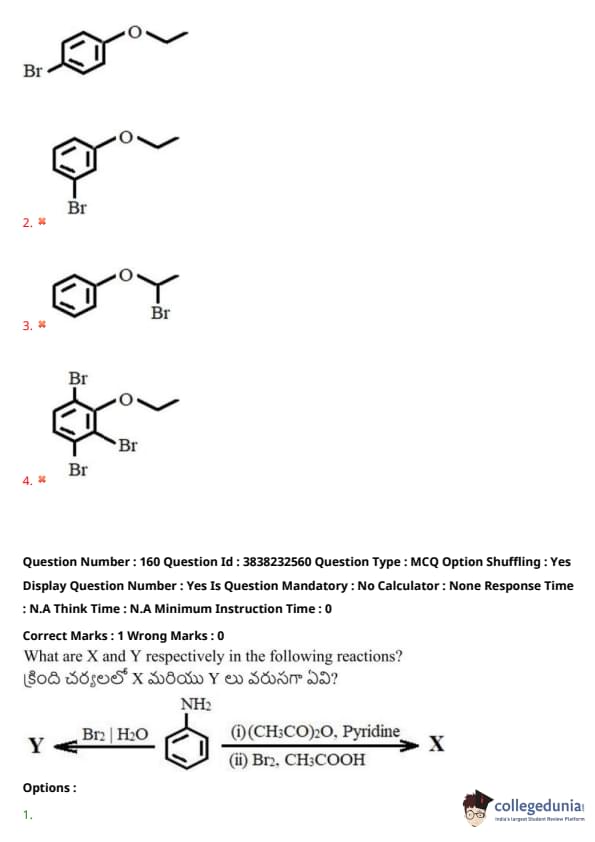
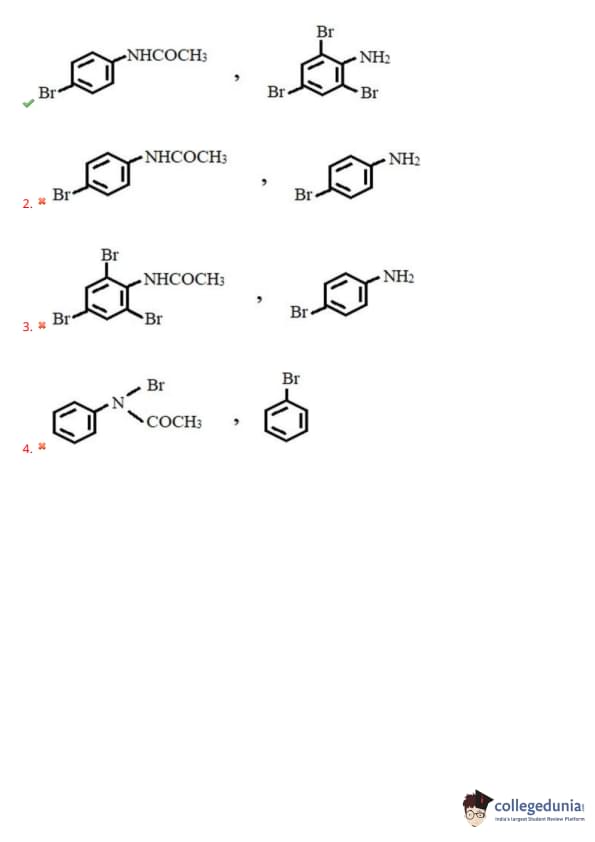
What are X and Y respectively in the following reactions?
View Solution












































































































Also Check:
TS EAMCET Previous Year Question Papers
| TS EAMCET 2023 Question Paper | TS EAMCET 2022 Question Paper |
| TS EAMCET 2021 Question Paper | TS EAMCET 2020 Question Paper |
| TS EAMCET 2019 Question Paper | TS EAMCET 2018 Question Paper |
Also Check:



Comments