TS EAMCET 2024 Question Paper May 7 Shift 1 with Answer Key PDF is available here. JNTU, Hyderabad on behalf of TSCHE conducted TS EAMCET on May 7 from 9 AM to 12 PM. TS EAMCET 2024 Question Paper consists of 160 questions carrying 1 mark each. TS EAMCET 2024 Question Paper May 7 Shift 1 PDF for BiPC includes four subjects, Physics, Chemistry and Biology with Botany & Zoology. Each subject includes 40 questions.
Candidates can use the link below to download the TS EAMCET 2024 Question Paper with detailed solutions.
TS EAMCET 2024 Question Paper with Answer Key May 7 Shift 1 PDF
| TS EAMCET 2024 Question Paper with Answer Key | Check Solution |
TS EAMCET Questions with Solutions
Botany
Question 1:
Flask-shaped ascocarp with an apical opening is:
View Solution
In which of the following organisms, cell wall is not found in any stage?
View Solution
Assertion (A): In Rhodophyceae, food is stored as floridean starch.
Reason (R): Floridean starch is similar to amylopectin and glycogen in structure.
View Solution
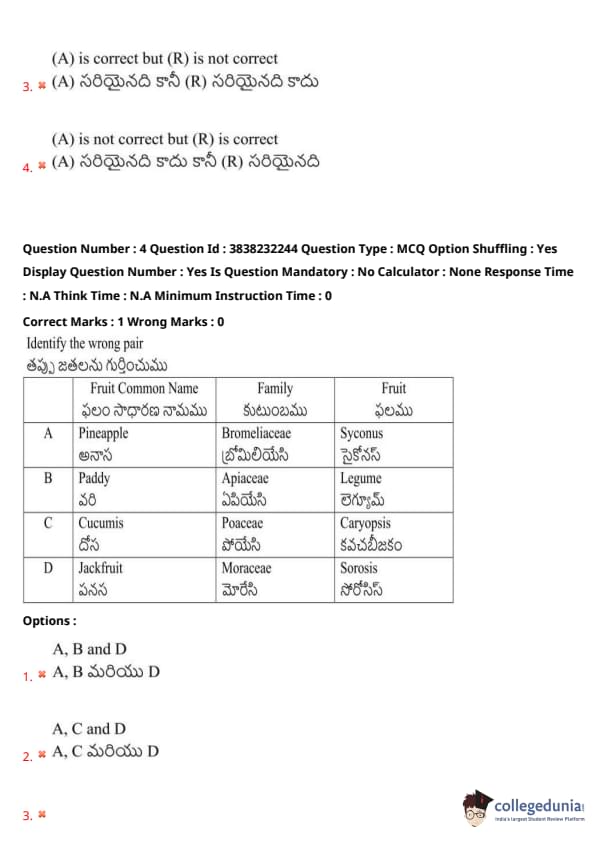
Identify the wrong pair:
View Solution
Match the following:
View Solution
Genetic nature of Ribonucleic acid was discovered by:
View Solution
Variation in lengths of filaments of stamens within a flower is seen in:
View Solution
Assertion (A): Direct pollination is found in gymnosperms.
Reason (R): Ovules of gymnosperms are naked.
View Solution
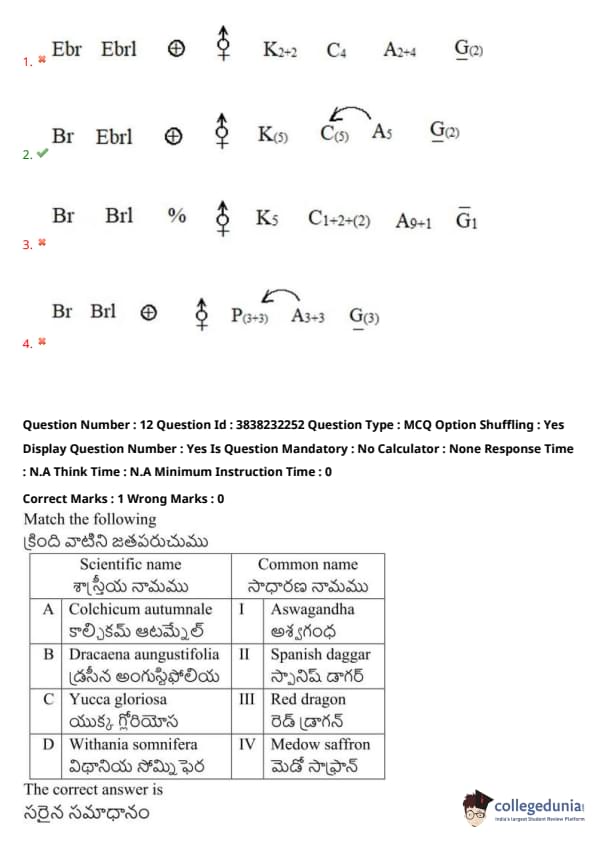
Match the following:
View Solution
Ategmic, Unitegmic, and Bitegmic ovules are present serially in:
View Solution
Floral formula of Solanum nigrum:
Match the following:

View Solution
In one helix of DNA, minimum and maximum number of hydrogen bonds present between nitrogen bases:
View Solution
The "R" group amino acids of Glycine, Alanine, and Serine respectively are:
View Solution
Cell organelles included in the endomembrane system are:
View Solution
Dehydration occurs during the formation of the following types of bonds:
View Solution
Match the following:
View Solution
The site of transcription and translation in eukaryotic cells are respectively:
View Solution
Specialized epidermal cells surrounding the guard cells are:
View Solution
Identify the correct statement with reference to the diagram given below:
View Solution
Identify the incorrect pair:
View Solution
Assertion (A): Disulphide bridges help in stabilization of protein structure.
Reason (R): Sulphur forms disulphide bridges in the quaternary structure of proteins.
View Solution
Match the following:
View Solution
Per one Calvin cycle, the number of CO\(_2\) molecules fixed, ATP utilized, and the number of Glucose, NADPH molecules produced respectively are:
View Solution
In C\(_4\) plants, the Oxaloacetic acid is formed by this reaction:
View Solution
Identify the correct sentences regarding the role of physiological responses of phytohormones:
I. Ethylene initiates sprouting of potato tubers
II. Gibberellins promote senescence
III. Auxins promote flowering in pineapple
IV, Abscisic acid inhibits seed germination
View Solution
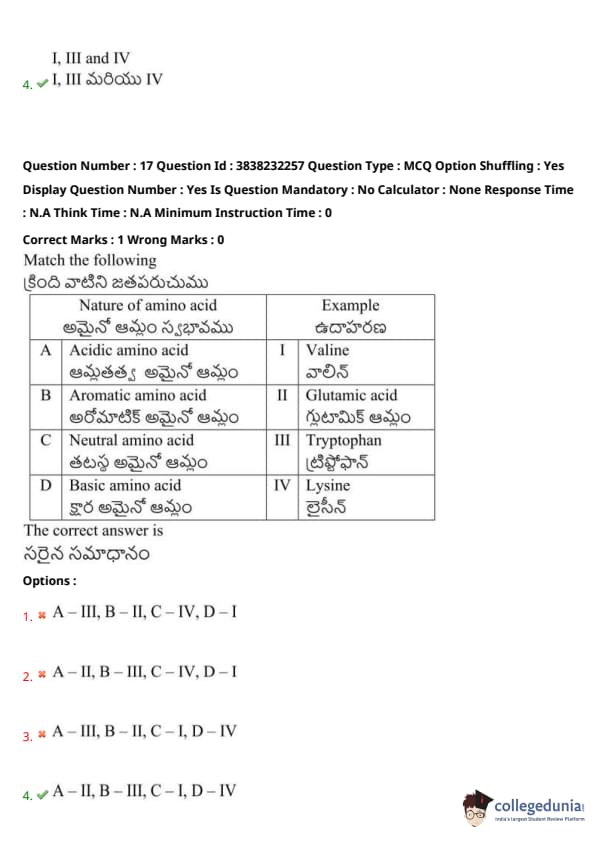
Match the following:
View Solution
Substrate-level phosphorylation does not occur in the following reactions of aerobic respiration:
View Solution
Match the following:
View Solution
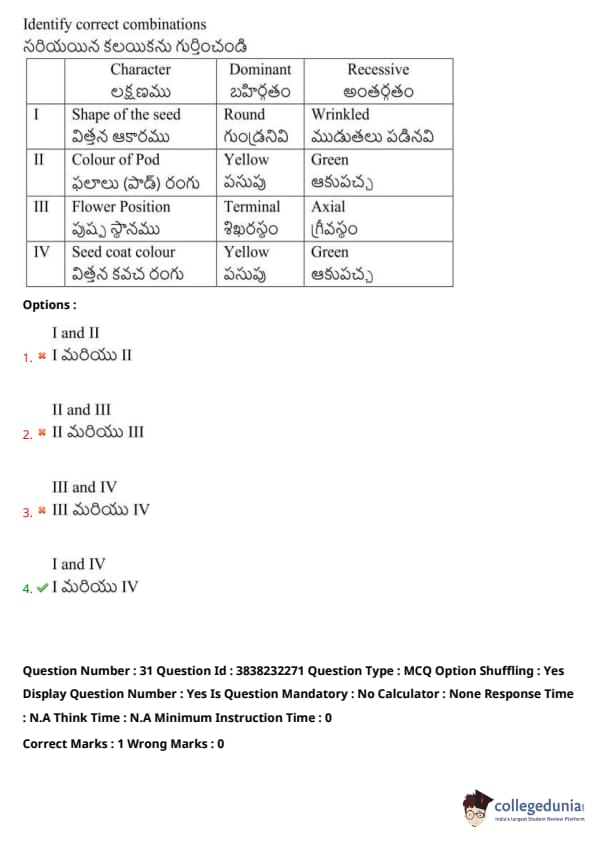
Identify the correct combinations:
View Solution
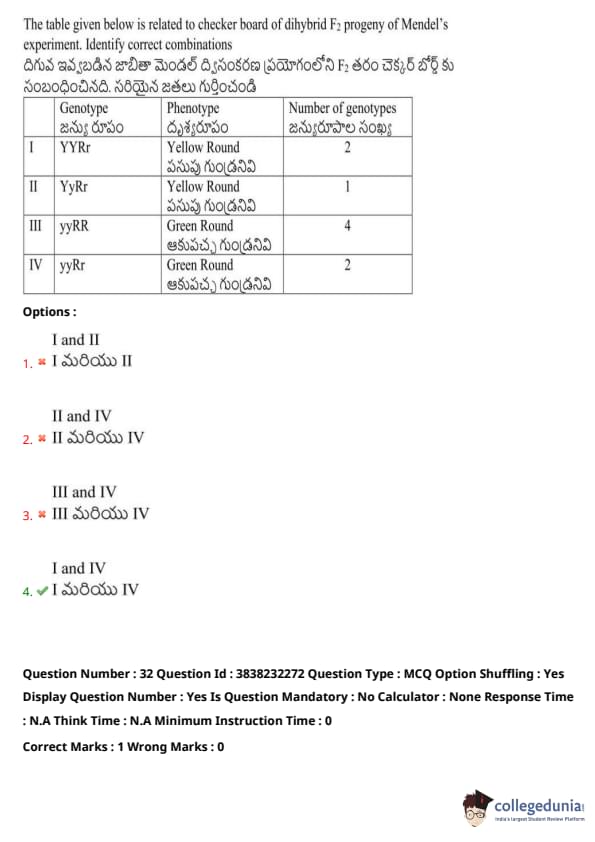
The table given below is related to the checkerboard of dihybrid F\(_2\) progeny of Mendel’s experiment. Identify the correct combinations:

View Solution
Assertion (A): Beggiatoa is not a chemoautotrophic bacterium.
Reason (R): Chemoautotrophic bacteria derive carbon from CO\(_2\) and energy from oxidation of inorganic compounds.
View Solution
In Morgan’s experiments on linkage in Drosophila, the percentage of white-eyed, miniature-winged recombinants in the F\(_2\) generation is:
View Solution
Match the following:

View Solution
Identify the correct definitions of the following molecular biology terms:
I. Splicing = Removal of introns
II. Capping = Addition of unusual methyl guanosine triphosphate added to 5" end of hn RNA
III. Tailing = Deletion of adenylate residues at 3'end of hn RNA
IV. Anticodon= Specifies termination of polynucleotide chain
View Solution
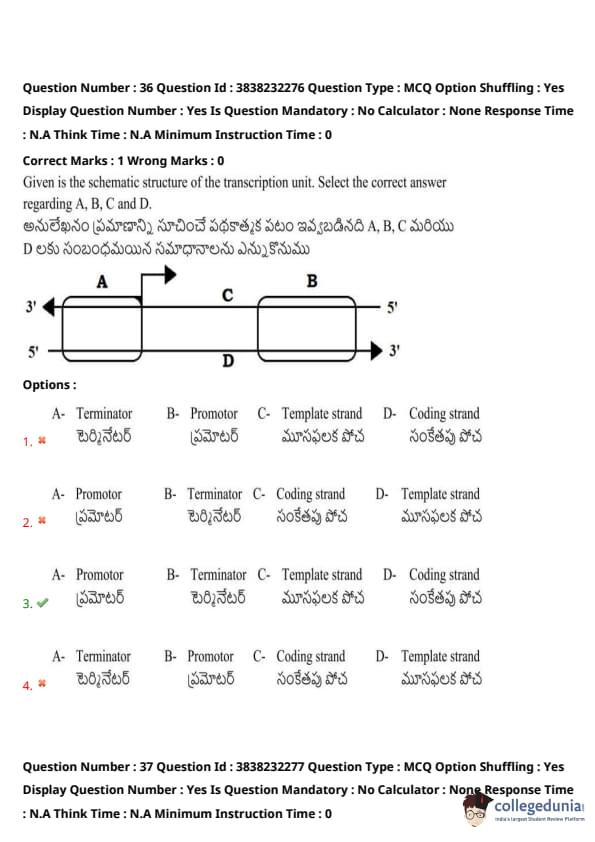
Given is the schematic structure of the transcription unit. Select the correct answer regarding A, B, C, and D:
View Solution
Tetracycline resistance gene of pBR322 consists of this restriction site:
View Solution
Assertion (A): In gel electrophoresis, DNA fragments get separated and move toward the anode.
Reason (R): DNA fragments are positively charged molecules.
View Solution
Thermostable DNA polymerase (Taq Polymerase) enzyme is isolated from the following bacterium:
View Solution
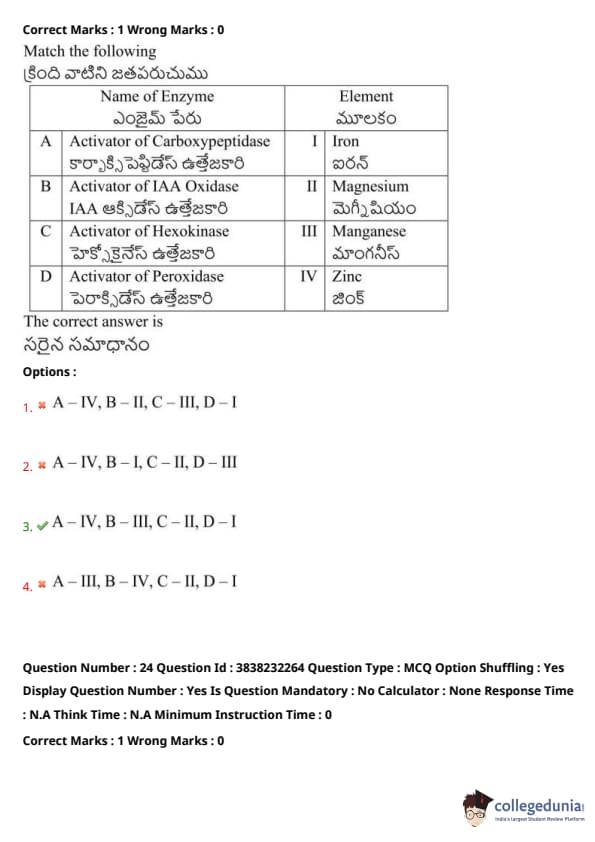
Match the following:

View Solution
Zoology
Question 41:
Study the following and pick the correct statements:
View Solution
Maintenance of relatively constant internal conditions different from the surrounding environment is called:
View Solution
Assertion (A): Some adult gastropods (e.g., snail) are asymmetrical.
Reason (R): Torsion takes place during the development of gastropods.
View Solution
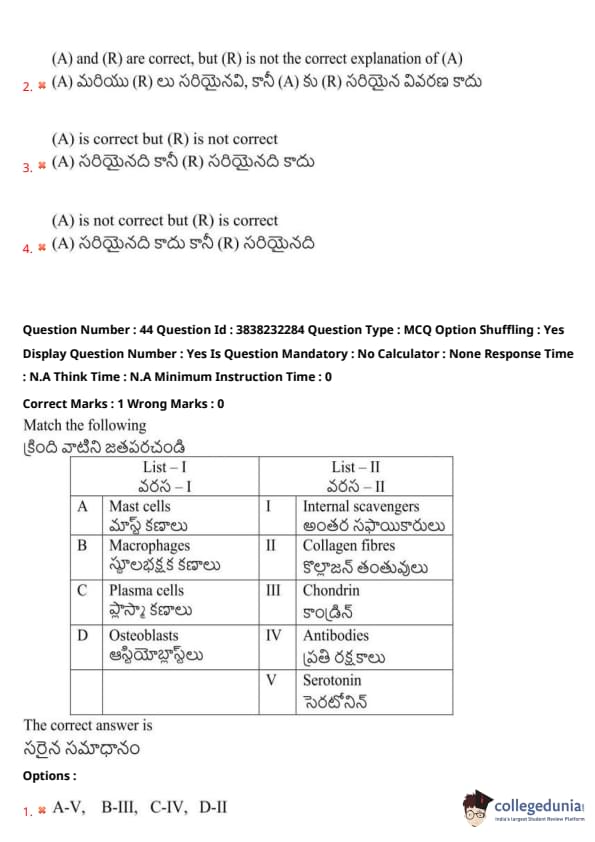
Match the following:

View Solution
Symmetry of sea anemone is:
View Solution
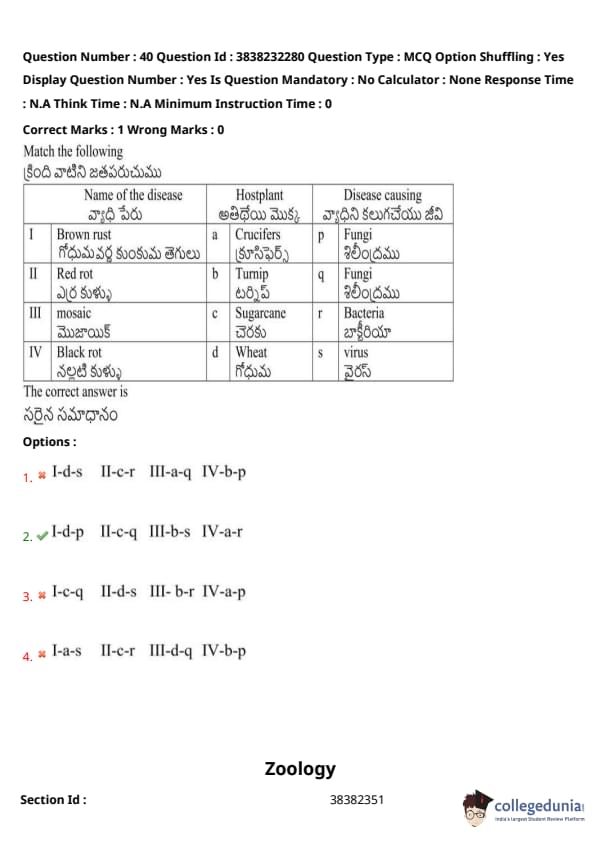
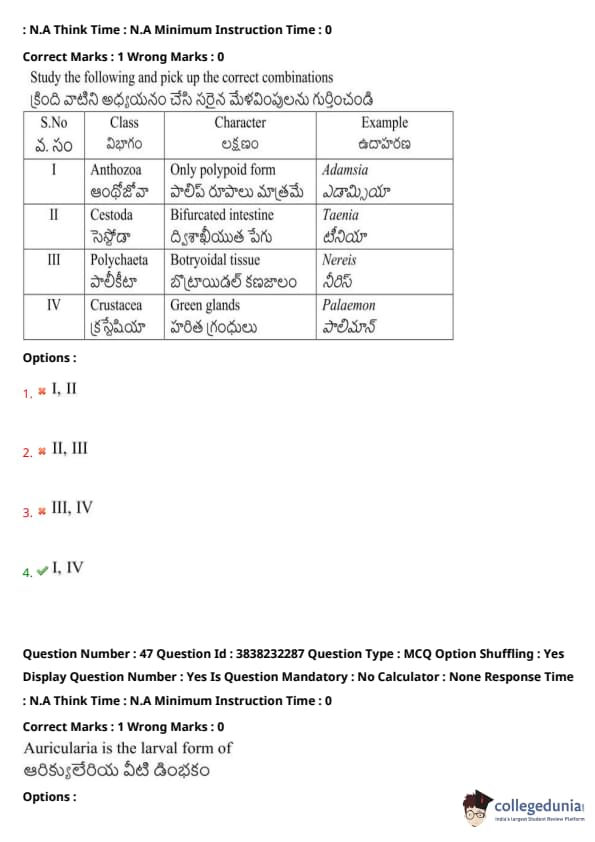
Study the following and pick up the correct combinations:

View Solution
Auricularia is the larval form of:
View Solution
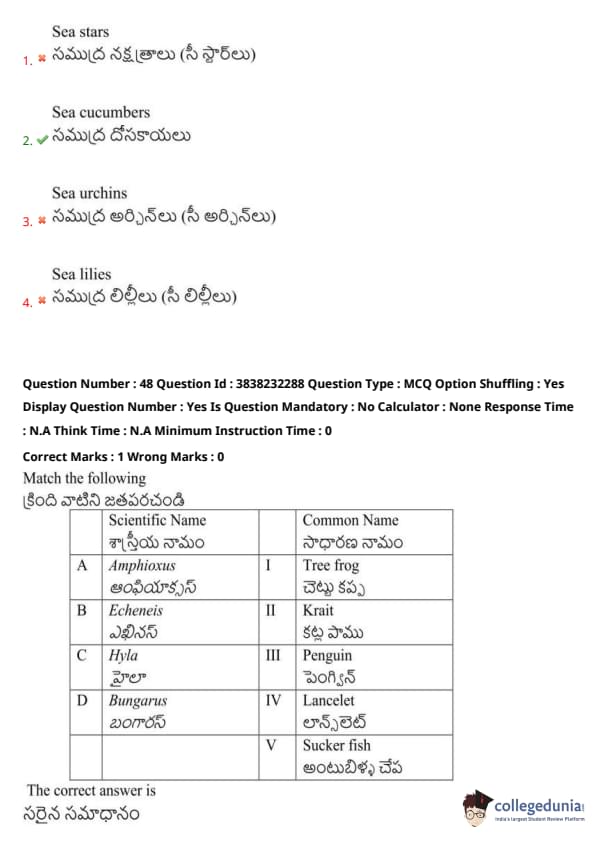
Match the following:

View Solution
Number of cervical vertebrae in most mammals is:
View Solution
Flagellum in Peranema is:
View Solution
Study the following and pick up the correct statements:
View Solution
Assertion (A): Wuchereria bancrofti is an intercellular parasite.
Reason (R): It lives among the cells of the tissues of the host.
View Solution
In man, Microsporum causes:
View Solution
It interferes with the transport of the neurotransmitter dopamine:
View Solution
In cockroach, wings are elevated by the contraction of:
View Solution
In cockroach, inspiration takes place through:
View Solution
In an experiment, Malpighian tubules of a cockroach are removed. Which substance might be absent in its fecal pellets?
View Solution
The influence of light on non-directional movement of organisms is known as:
View Solution
Statement I: Acid rains are due to pollution of sulphur dioxide and nitrogen oxides.
Statement II: Incinerators are used to dispose of electronic wastes.
View Solution
Match the following:

View Solution
The enzyme present in the gastric juice of infants is:
View Solution
Assertion (A): During inspiration, the rib cage and sternum lift up, causing an increase in the volume of the thoracic cavity in the dorso-ventral axis.
Reason (R): Due to relaxation of the diaphragm muscles, the volume of the thoracic cavity increases.
View Solution
Statement I: In the foetal heart of human beings, the interatrial septum has a pore called foramen Manro.
Statement II: A fibrous strand, known as ligamentum arteriosum, is present at the point of contact of the systemic and pulmonary arches in the human heart.
View Solution
Excretory organs in adult molluscs are:
View Solution
Regulatory proteins in a myofibril are:
View Solution
Study the following and pick up the correct statements:
The center of the posterior portion of the retina is called the macula lutea.
The site of the retina where the optic nerve exits the eye is called the blind spot.
Cones contain a visual pigment called iodopsin.
Rods contain a protein called rhodopsin.
View Solution
Study the following and pick up the correct combinations:

View Solution
Chemically, oestrogens (oestradiol) are:
View Solution
Match the following:

View Solution
These glands of the female reproductive system of human beings are homologous to the bulbourethral glands of males.
View Solution
Pick up the incorrect pair.
View Solution
If a man with blood group A (homozygous) marries a woman with blood group B (homozygous), the following blood groups are not expected in their children.
View Solution
Karyotype of Klinefelter’s syndrome is:
View Solution
If sex index ratio in Drosophila is 0.33, the sexual phenotype of it is:
View Solution
Eusthenopteron is a transitional form between:
View Solution
Statement I: Absence of gene exchange between populations is called reproductive isolation.
Statement II: Natural selection is the driving force of evolution.
View Solution
Hardy-Weinberg law is applicable to:
View Solution
Assertion (A): Continued close breeding usually reduces fertility and even productivity.
Reason (R): Inbreeding depression.
View Solution
In an ECG of a normal healthy person, the duration of the R-R interval is about:
View Solution
Match the following:

View Solution
Physics
Question 81:
A supposition without assuming that it is true is:
View Solution
The density of a substance of mass 5.318 g which occupies a volume of 2.43 cm³ is (up to correct significant figures):
View Solution
Pebbles are dropped freely for every half second into a river from a bridge above it. When the first pebble is about to strike the surface of the water, the fifth pebble is dropped. Then the height from the surface of the water from which the pebbles are dropped is (Acceleration due to gravity = 10 m/s²).
View Solution
A cannon fires two similar shells one after the other each with a velocity of 100 m/s at angles 60\(^\circ\) and 30\(^\circ\) respectively with the horizontal such that they both hit the target at the same time. The time interval between the firing of the two shells is (Acceleration due to gravity = 10 m/s²).
View Solution
A 60 kg man standing on a bridge, jumps vertically down onto a 540 kg boat moving in the river below him with a speed of 10 m/s. The change in the speed of the boat is:
View Solution
A body of mass 'm' tied to one end of a string is whirled in a vertical circle of radius 'R' with zero tension in the string at its highest point. The angle made by the string with the vertical when the kinetic energy of the body becomes half of its maximum kinetic energy is:
View Solution
An incompressible fluid is flowing through a tube of uniform cross-section. The increase in the power of the pump required to double the rate of flow is:
View Solution
Two solid spheres of radii \( r_1 \) and \( r_2 \) (\( r_2 > r_1 \)) made of the same material are kept in contact. The distance of their center of mass from their point of contact is:
View Solution
An arc making an angle \(15^\circ\) at the center is removed from a ring of mass \( M \) and radius \( R \). The moment of inertia of the remaining ring about an axis passing through its center and perpendicular to its plane is:
View Solution
The displacement of a particle in simple harmonic motion is given by:
\[ x = A_0 \cos \left( \frac{\pi}{2} t \right) \]
The distance traveled by the particle in the interval between \( t = 2 \) and \( t = 5 \) seconds and its position at \( t = 5 \) second are:
View Solution
The ratio of the values of acceleration due to gravity at heights \( h_1 \) and \( h_2 \) from the surface of the earth is \( 16:9 \). If height \( h_1 = 2R_E \), then \( h_2 \) is:
View Solution
The ratio of the lengths of two wires A and B made of same material is 2:1. The diameter of wire A is twice the diameter of wire B. If both the wires are stretched by same tension, the ratio of the energies stored in wires A and B is:
View Solution
Two spherical rain drops of radii in the ratio 4:5 are falling vertically through air. The ratio of the terminal velocities of the rain drops is:
View Solution
A liquid drop of diameter 2 mm breaks into 125 identical drops, then the change in the surface energy is:
View Solution
A metal block of mass 120 g is heated to a temperature of 100\(^\circ\)C and placed on a huge block of ice at 0\(^\circ\)C. The specific heat capacity of the metal is 0.12 cal/\(^\circ\)C and the latent heat of fusion of ice is 80 cal/g. The mass of the ice melted is:
View Solution
A black body at a temperature of 125\(^\circ\)C emits heat at the rate of 32 W. The rate of heat emitted by the body when the temperature of the body is increased by 398 K is:
View Solution
The temperatures of the source and sink of a Carnot’s heat engine are 27\(^\circ\)C and 127\(^\circ\)C respectively. If the absolute temperature of the sink is decreased by 10%, the efficiency of the engine:
View Solution
A uniform narrow tube of length one metre with one end closed contains 25 cm long mercury thread, which traps a column of air at the closed end. When the tube is held vertically with the open end up, the length of the air column near the closed end is 21 cm and when the tube is held horizontally, the length of the air column near the closed end is ‘L’. If the atmospheric pressure is equal to the pressure of 75 cm of mercury, then \( L \) is:
View Solution
If the tension applied to a string is decreased by 36%, then the fundamental frequency of the transverse waves of the string is:
View Solution
The frequency of sound heard by a stationary observer is \( f_1 \), when the source of sound is approaching the observer with a speed of 10% of the speed of sound. If the same source of sound is moving away from the stationary observer with a speed of 20% of the speed of sound, the frequency of sound heard by the observer is \( f_2 \). Then \( f_1 : f_2 \) is:
View Solution
A boy of height 1.2 m is standing in front of a large concave mirror of radius of curvature 20 m at a distance of 40 m from the mirror. The distance of the image of the boy from the boy is:
View Solution
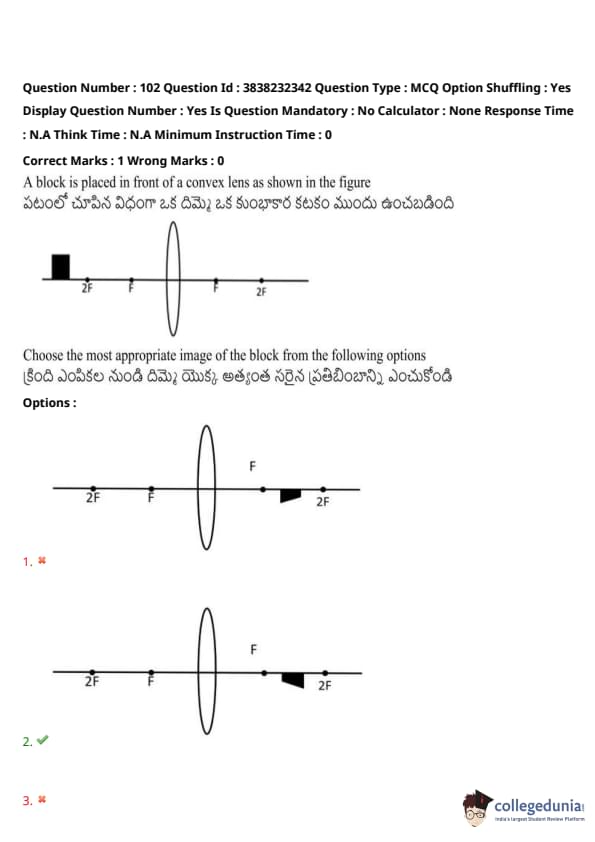
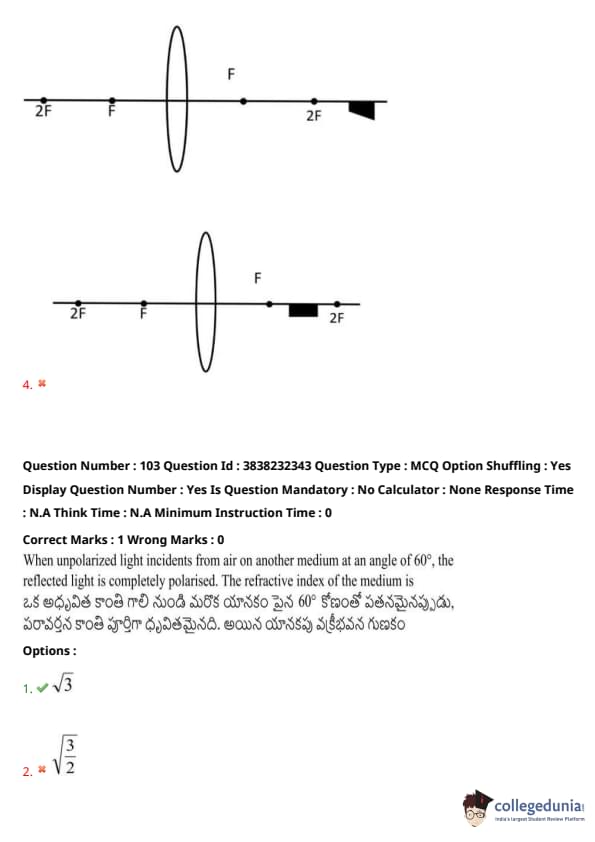
A block is placed in front of a convex lens as shown in the figure. Choose the most appropriate image of the block from the following options:
View Solution
When unpolarized light incident from air on another medium at an angle of 60°, the reflected light is completely polarized. The refractive index of the medium is:
View Solution
Two identical balls having like charges placed at a certain distance apart, repel each other with a force \( F \). When they are brought in contact and then moved apart to a distance equal to half of their initial separation, the force of repulsion between them becomes \( 4.5F \). The ratio of the initial charges of the balls is:
View Solution
The capacitance of a parallel plate capacitor is 1.5 µF. If the distance between the plates is halved and the space between the plates is filled with a medium of dielectric constant 3, then the new capacitance is:
View Solution
If the relaxation time is doubled and the applied electric field is tripled, then the drift speed of electrons:
View Solution
Two equal resistances are connected in the two gaps of a meter bridge. If the resistance in the right gap is doubled, then the change in the balancing length is:
View Solution
A current \( i \) flows through a circular loop of radius \( R \). The ratio of the magnetic field produced at its centre to the field produced at a point at a distance \( \frac{R}{\sqrt{3}} \) from its centre on its axis is:
View Solution
Two particles of charges in the ratio \( 1:2 \) and masses in the ratio \( 2:3 \) moving along a straight line enter a uniform magnetic field at right angles to the direction of the field. If the radii of the circular paths of the particles in the magnetic field are in the ratio \( 3:4 \), then the ratio of the initial linear momenta of the two particles is:
View Solution
Magnetic field on the axis of a short bar magnet of magnetic moment \( M \), at a distance \( x \) from its centre is:
View Solution
The self-induced emf of a coil is 36 V. If the current in the coil is changed from 12 A to 24 A in one second, then the change in the energy stored in the coil is:
View Solution
The quality factor of a series LCR resonant circuit is 75. If the resistance is decreased by 50% and the inductance is increased by 100%, then the quality factor of the circuit is:
View Solution
The ratio between electric field energy density and magnetic field energy density of an electromagnetic wave, in its region is:
View Solution
Two photons of energies 2.5 eV and 5.5 eV incident on a metal surface of work function 1.5 eV. The ratio of the maximum speeds of the photoelectrons emitted from the metal surface is:
View Solution
In Bohr model of hydrogen atom, if the difference between the radii of \( n^{th} \) and \( (n+1)^{th} \) orbits is equal to the radius of the \( (n-1)^{th} \) orbit, then the value of \( n \) is:
View Solution
In a nuclear reactor of efficiency 25%, the number of fissions taking place per second is \( 5 \times 10^{13} \). If 200 MeV energy is released per fission, then the output power of the reactor is:
View Solution
In a radioactive sample \( 2 \times 10^8 \) nuclei reduce to \( 10^8 \) nuclei in 15 minutes, then the half-life of the sample in minutes is:
View Solution
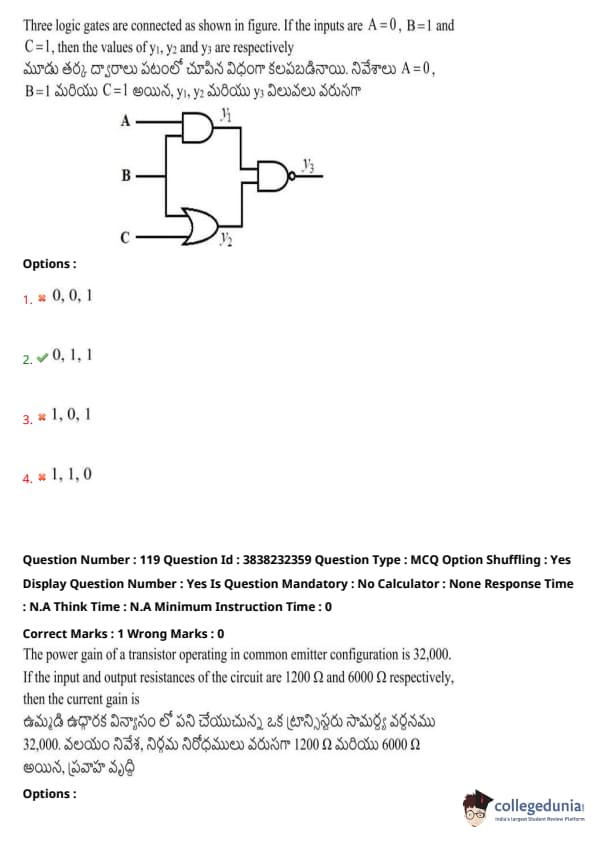
Three logic gates are connected as shown in the figure. If the inputs are \( A = 0 \), \( B = 1 \), and \( C = 1 \), then the values of \( y_1 \), \( y_2 \), and \( y_3 \) are respectively:
View Solution
The power gain of a transistor operating in common emitter configuration is 32,000. If the input and output resistances of the circuit are 1200 \( \Omega \) and 6000 \( \Omega \) respectively, then the current gain is:
View Solution
If two linear antennas having lengths in the ratio 2:3 are emitting radiations of wavelengths in the ratio 8:9, then the ratio of effective powers radiated by them are in the ratio:
View Solution
Chemistry
Question 121:
Electromagnetic radiation has electric and magnetic field components. These two components:
View Solution
The wavenumbers of the first three emission lines of Lyman series of hydrogen spectrum are respectively \( \nu_1, \nu_2, \nu_3 \). Similarly, the wavenumbers of first three emission lines of Balmer series of hydrogen spectrum are \( \nu_4, \nu_5, \nu_6 \), respectively. Identify the correct relationship:
View Solution
The first ionization enthalpies of the elements X, Y, Z of the second period are 899, 1402, 520 kJ/mol respectively. X, Y, Z respectively are:
View Solution
The number of amphoteric, basic, and acidic oxides among the following respectively are: \[ CrO_3, \, MgO, \, K_2O, \, B_2O_3, \, Al_2O_3, \, In_2O_3, \, PbO, \, As_2O_3 \]
View Solution
Identify the correct statements:
(i) LiF has more covalent character compared to KF
(ii) Dipole moment of \( NF_3 \) is greater than that of \( NH_3 \)
(iii) The bond order is same for \( F_2 \) and \( O_2 \)
(iv) Ionic compounds possess low melting and boiling points
View Solution
Which of the following sets are not correctly matched? \[ Molecule \quad Hybridization \] \[ (i) \, XeF_4 \quad dsp^2 \quad (ii) \, BrF_5 \quad sp^3d^2 \quad (iii) \, PF_5 \quad sp^3d \quad (iv) \, SF_4 \quad sp^3 \]
View Solution
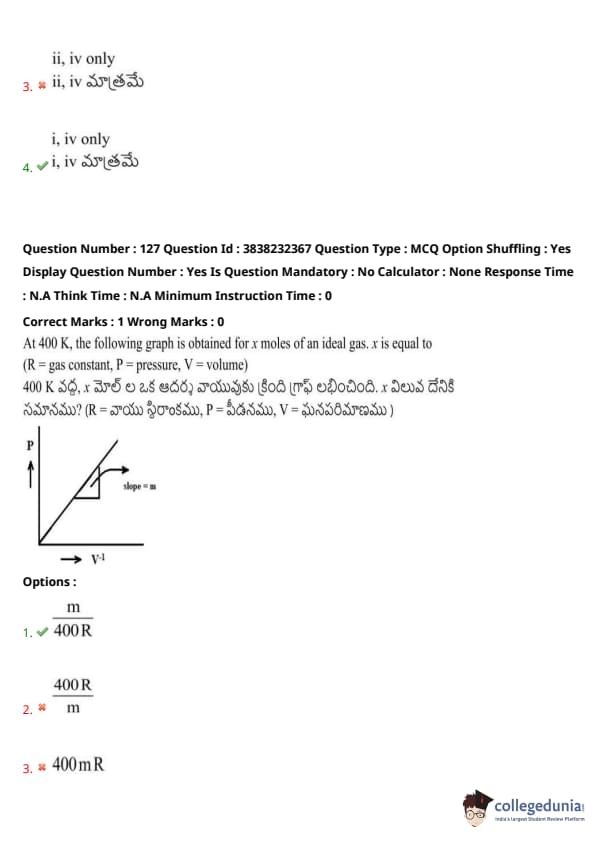
At 400 K, the following graph is obtained for \( x \) moles of an ideal gas. \( x \) is equal to (R = gas constant, P = pressure, V = volume)
View Solution
In Ostwald process of manufacture of nitric acid, 12 moles of NH3 was completely oxidized in air by a catalyst at 500 K and 9 bar. The resultant NO(g) was completely oxidized to NO2(g) and dissolved in water to form nitric acid and NO(g). What is the weight (in g) of nitric acid formed?
View Solution
Observe the following two statements:
I. For an isolated system, \( \Delta U = 0 \); \( q = 0 \)
II. For a closed system, \( \Delta U = 0 \); \( q \neq 0 \)
The correct answer is
View Solution
For the reaction at \( T(K) \), \( A_2(g) \rightleftharpoons B_2(g) \), \( K_c \) for the reaction at \( T(K) \) is 39.0. In a closed 1L flask, one mole of \( A_2(g) \) was heated to \( T(K) \). What is the concentration of \( B_2(g) \) at equilibrium (in mol L\(^{-1}\))?
View Solution
Two statements are given below:
I. Sodium hexametaphosphate is used in calgon method for removal of permanent hardness of water
II. Lithium forms interstitial hydrides
Identify the correct answer
View Solution
The correct order of reducing power of alkali metals in aqueous solution is
View Solution
Observe the following statements
Statement I : Ca(OH)\(_2\); is used in white wash
Statement 11 : \(CaCO_3\) is used as mild abrasive in tooth paste
View Solution
Identify the correct statements from the following:
I. \(In_2O_3\) is a basic oxide.
II. \(TiCl_2\) is more ionic in nature than \(TiCl_3\).
III. Boron reacts with dinitrogen at high temperature to form BN.
View Solution
In group 14 elements, the element with highest melting point is X and element with lowest melting point is Y. X and Y respectively are
View Solution
The oxygen carrying capacity of blood is reduced due to binding of haemoglobin with
View Solution
The technique used to purify an organic compound present in aqueous medium and which is less soluble in organic solvent is
View Solution
The number of molecules with electrophilic centres in the following is
CH\(_3\)CH\(_2\)Br, CH\(_3\)COCH\(_3\), CH\(_3\)CH\(_2\)CN, (CH\(_3\))\(_2\)Cd
View Solution
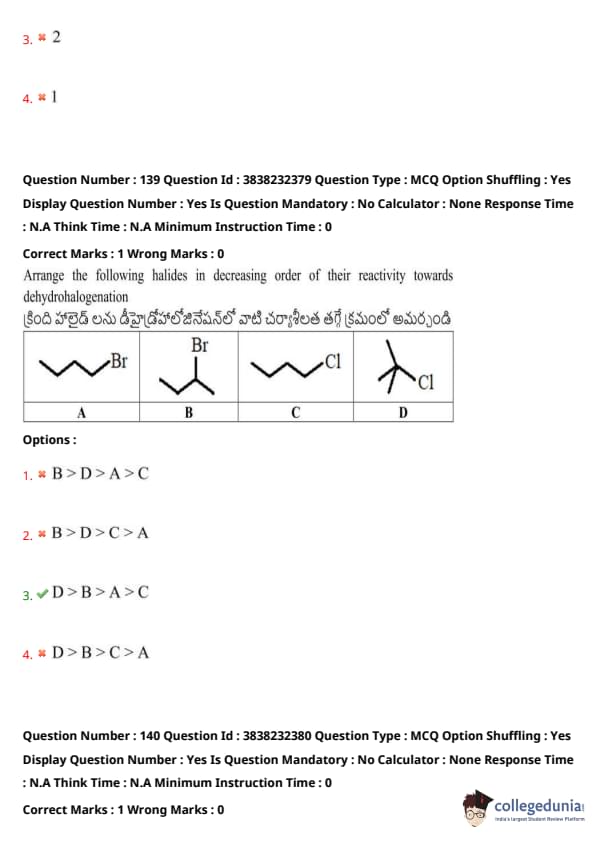
Arrange the following halides in decreasing order of their reactivity towards dehydrohalogenation:
View Solution
An alkene ‘X’ (C\(_4\)H\(_8\)) does not exhibit cis/trans isomerism. Reaction of ‘X’ with Br\(_2\)/CCL\(_4\), followed by reaction with reagent ‘Y’ gave ‘Z’ (C\(_6\)H\(_6\)). What are ‘Y’ and ‘Z’ respectively?
View Solution
Identify the correct set
View Solution
At 300 K, the vapour pressure of liquids A and B are 600 and 500 mm of Hg respectively. Two moles of A and three moles of B are mixed. The mole fractions of A and B in vapor state are respectively
View Solution
What is the electrode potential (in V) of copper electrode dipped in \( 10^{-2} \, M \, Cu^{2+} \) solution?
View Solution
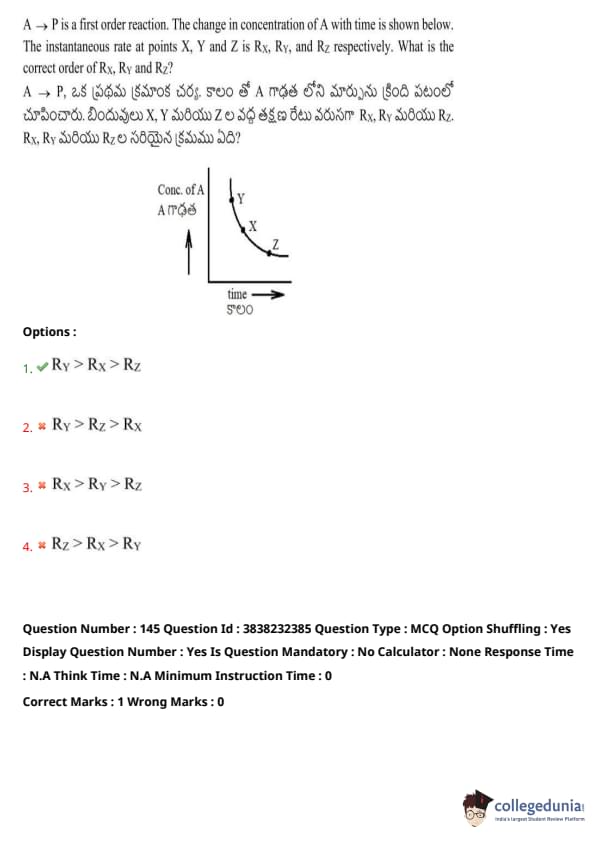
A \(\rightarrow\) P is a first-order reaction. The change in concentration of A with time is shown below. The instantaneous rate at points X, Y and Z is \( R_X \), \( R_Y \), and \( R_Z \), respectively. What is the correct order of \( R_X \), \( R_Y \), and \( R_Z \)?
View Solution
Two statements are given below.
Statement I: Adsorption of a gas on the surface of charcoal is primarily an exothermic process.
Statement II: A closed vessel containing \( O_2(g) \), \( H_2(g) \), \( Cl_2(g) \), \( NH_3(g) \) has a pressure of 9 atm. About 1 g of charcoal was added to this vessel and after some time its pressure is \( P' \) atm. It is observed that \( P' > P \).
Choose the correct answer.
View Solution
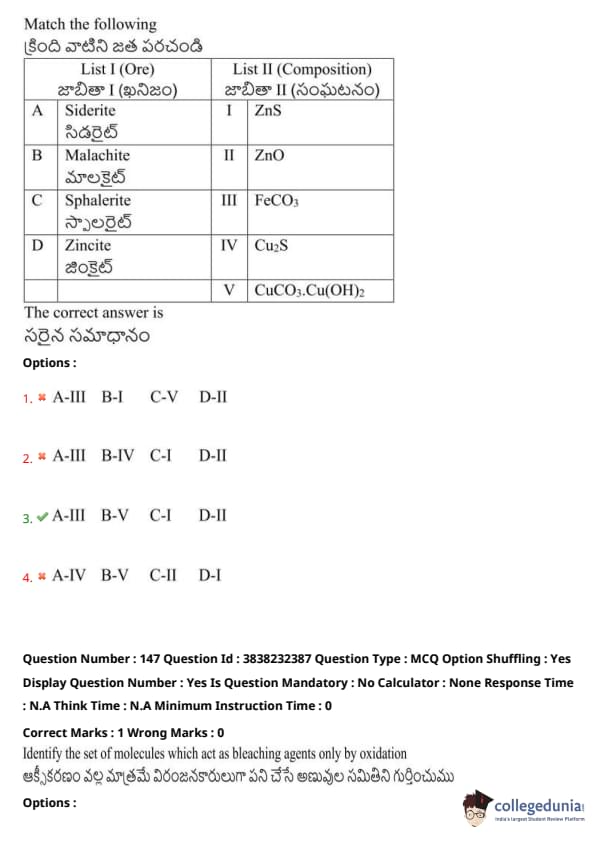
Match the following:

View Solution
Identify the set of molecules which act as bleaching agents only by oxidation
View Solution
Which of the following is correct statement?
View Solution
Which of the following does not evolve \( O_2 \) when made to react with water?
View Solution
Observe the following reaction:
\[ xI^- + y \, \, MnO_4^- + z \, H^+ \rightarrow a \, Mn^{2+} + b \, H_2O + cl_2 \]
Which of the following are correct?
(i) \( y : x = 2 : 5 \)
(ii) \( y : a = 1 : 1 \)
(iii) \( x : c = 1 : 2 \)
(iv) \( y : e = 2 : 5 \)
View Solution
Which of the following are inner orbital paramagnetic complexes?
View Solution
The number average molecular weight (M\(_n\)) of a polymer is 1500. In this polymer, 800 molecules of molar mass 1000, 100 molecules of molar mass 2000 and x molecules of molar mass 5000 are present. What is the value of x?
View Solution
Phosphodiester linkage that joins nucleotides together is present between which carbons of pentose sugar?
View Solution
Which of the following is used in liquid detergents?
View Solution
Given below are the two statements regarding chlorobenzene
Statement I: Chlorobenzene is less reactive than benzene towards electrophilic substitution due to the -I effect of chlorine.
Statement II: Because of the -I effect of chlorine, it is a meta directing group.
View Solution
Which of the following sequence of reagents convert benzaldehyde to 4-chlorotoluene?
View Solution
Phenatole can be prepared from which of the following reactants?
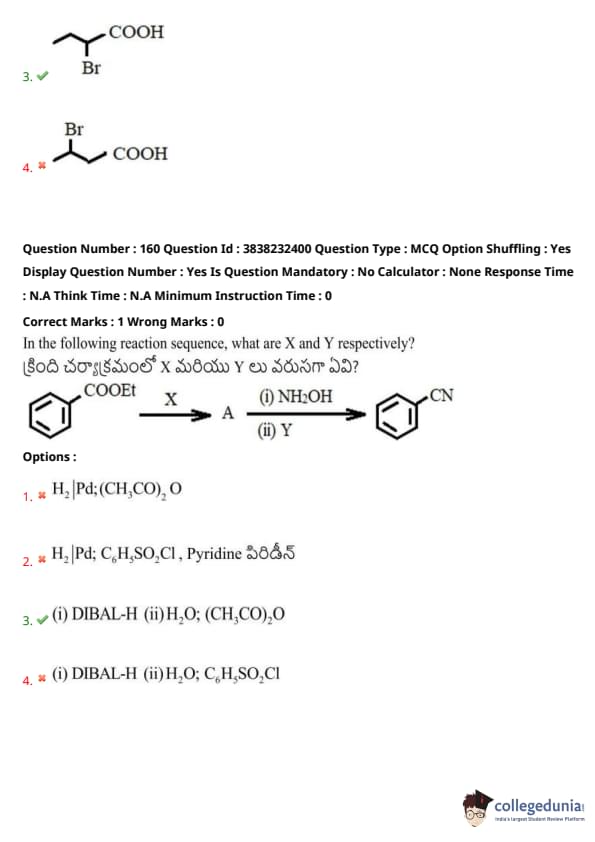
In the following reaction sequence, what are X and Y respectively?
Identify 'Z' in the following sequence of reactions:
In the following reaction sequence, what are X and Y respectively?
View Solution











































































































Also Check:
TS EAMCET Previous Year Question Papers
| TS EAMCET 2023 Question Paper | TS EAMCET 2022 Question Paper |
| TS EAMCET 2021 Question Paper | TS EAMCET 2020 Question Paper |
| TS EAMCET 2019 Question Paper | TS EAMCET 2018 Question Paper |
Also Check:



Comments