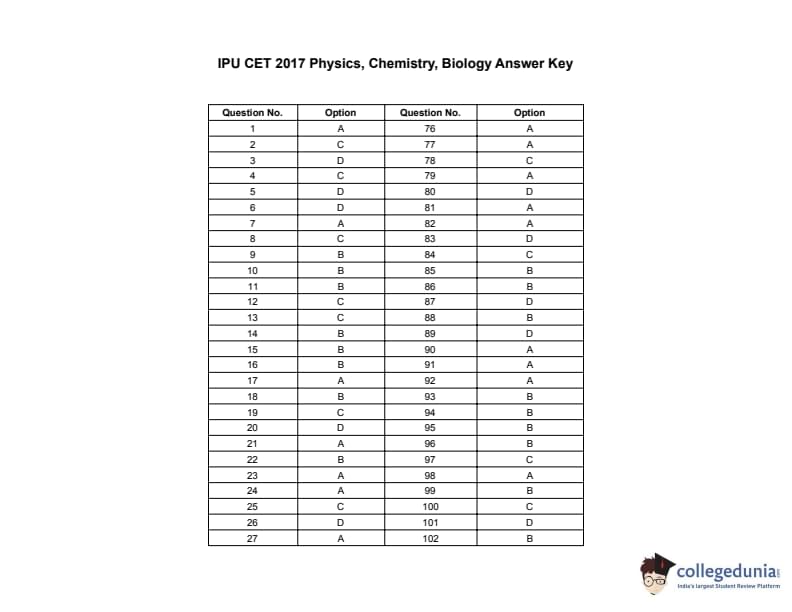
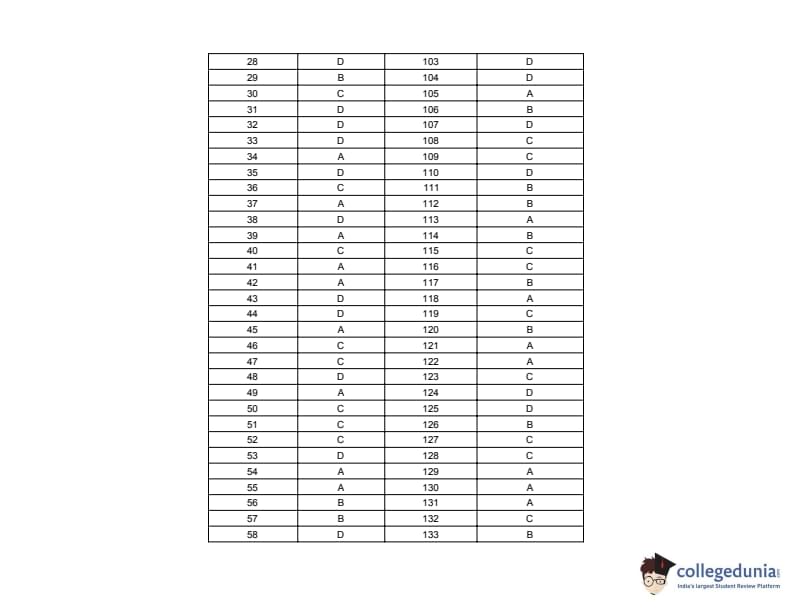
The IPU CET PCB exam is an entrance test for admission to various programs at Guru Gobind Singh Indraprastha University for students from the Physics, Chemistry, and Biology (PCB) stream. It consists of 100 questions covering subjects such as Physics, Chemistry, Biology, and General Awareness. Students can download here the IPU CET question papers along with solutions PDF.
IPU CET 2017 PCB Question Paper PDF Download
| IPU CET 2017 PCB Question Paper with Answer Key | Check Solution |

Question 1:
A candle C sits between two parallel mirrors at a distance \( 0.2d \) from mirror 1. Here \( d \) denotes the distance between the mirrors. Multiple images of the candle appear in both mirrors. How far behind mirror 1 are the nearest three images of the candle in that mirror?
A block of mass \( m \) is in contact with the cart C as shown in the figure. The coefficient of static friction between the block and the cart is \( \mu \). The acceleration \( a \) of the cart that will prevent the block from falling satisfies:
View Solution
If the escape speed of a projectile on Earth's surface is \( 11.2 \, km/s \) and a body is projected out with thrice this speed, then determine the speed of the body far away from the Earth.
View Solution
Consider the analogy between an oscillating spring-body system and an oscillating L-C-R circuit. Then, the correspondence between the two systems that is NOT correct is:
View Solution
A tank is filled with a liquid upto a height \( H \). A small hole is made at the bottom of this tank. Consider \( t_1 \) be the time taken to empty the first half of the tank and \( t_2 \) be the time taken to empty the rest half of the tank. Then, determine the ratio \( \frac{t_1}{t_2} \).
View Solution
In a cinema, a picture 2.5 cm wide on the film is projected to an image 3.0 m wide on a screen that is 18 m away. The focal length of the lens is about:
View Solution
The coefficient of volume expansion of glycerine is \( 49 \times 10^{-5} \, K^{-1} \). What is the fractional change in its density for a \( 30^\circ C \) rise in temperature?
View Solution
A capacitor of capacitance 5 µF is connected as shown in the figure. The internal resistance of the cell is 0.5 Ω. The amount of charge on the capacitor plate is:
View Solution
Use the diagram below to answer the following questions. 40 spheres of equal mass make two rings of 20 spheres each. The ring on the right has a radius twice as large as the ring on the left. At what position could a mass be placed so that the gravitational force it would experience would be the same from both rings?
View Solution
If pressure of CO\(_2\) (real gas) in a container is given by \( P = \frac{RT}{2v-b} - \frac{a}{4v^2} \), then mass of the gas in the container is:
View Solution
Two litres of water kept in a container at \( 27^\circ C \) is heated with a coil of 1 kW. The lid of the container is open and energy dissipates at the rate of 160 J/s. If the specific heat of water is 4.2 kJ/kg, then the time taken by coil to raise the temperature of water from \( 27^\circ C \) to \( 77^\circ C \) is:
View Solution
The resistance \( R \) of a conductor varies with temperature \( t \) as shown in the figure. If the variation is represented by \( R_t = R_0 \left( 1 + \alpha t + \beta t^2 \right) \), then:
View Solution
The bob of a pendulum is released from a horizontal position A as shown in the figure. If the length of the pendulum is 1.5 m, what is the speed with which the bob arrives at the lower most point B, given that it dissipated 5% of its initial energy against air resistance?
View Solution
A ring of radius \( R \) is first rotated with an angular velocity \( \omega \), and then carefully placed on a rough horizontal surface. The coefficient of friction between the surface and the ring is \( \mu \). Time after which its angular speed is reduced to half is:
View Solution
A ball of mass \( m \) moving at a speed \( v \) makes a head-on collision with an identical ball at rest. The kinetic energy at the balls after the collision is \( \frac{3}{4} \) of the original. What is the coefficient of restitution?
View Solution
A wire of arbitrary shape carries a current \( I = 2A \). Consider the portion of wire between \( (0, 0, 0) \) and \( (4, 4, 4) \). A magnetic field given by \[ B = \left( 1.2 \times 10^{-4} + 2 \times 10^{-4} \right) \, \hat{k} \]
\text{exists in the region. The force acting on the given portion of the wire is:
View Solution
The power of the combination of two lenses made by keeping the convex lens of focal length 40 cm in contact with the concave lens of focal length 25 cm, is:
View Solution
Two coherent point sources \( S_1 \) and \( S_2 \) vibrating in phase emit light of wavelength \( \lambda \). The separation between them is \( 2\lambda \). The light is collected on a screen placed at a distance \( D \gg \lambda \) from the slit \( S_1 \) as shown. The minimum distance, so that intensity at \( P \) is equal to intensity at \( O \), is:
View Solution
What will be the displacement equation of the simple harmonic motion obtained by combining the motions?
Given: \[ x_1 = 2 \sin(\omega t), \quad x_2 = 4 \sin(\omega t + \frac{\pi}{2}), \quad x_3 = 6 \sin(\omega t) \]
View Solution
A man holds a rectangular card in front of and parallel to a plane mirror. In order for him to see the entire image of the card, the least mirror area needed is:
View Solution
A stream of water flowing horizontally with the speed of 15 m/s gushes out of a tube of cross-sectional area \( 10^{-2} \, m^2 \) and hits a vertical wall nearby. What is the force exerted on the wall by the impact of water? Assuming it does not rebound.
View Solution
The North pole of the Earth's magnetic field is at the geographical South pole. A compass is a small magnet whose North pole end is drawn in the approximate direction of
View Solution
A shell of mass 0.020 kg is fired by a gun of mass 100 kg. If the muzzle speed of the shell is 80 m/s, what is the recoil speed of the gun?
View Solution
An electron of a stationary hydrogen atom passes from the fifth energy level to the ground level. The velocity that the atom acquired as a result of photon emission will be:
View Solution
If torques of equal magnitudes are applied to a hollow cylinder and a solid sphere both having the same mass and radius. The cylinder is free to rotate about its standard axis of symmetry and the sphere is free to rotate about an axis passing through its center. Which of the two will acquire a greater angular speed after a given time?
View Solution
Which of the following factors by itself will increase the frequency at which an observer hears a sound emanating from a source?
View Solution
If two simple pendulums first of bob mass \( M_1 \) and length \( l_1 \), and \( M_2 \) and \( l_2 \), Given \( M_1 = M_2 \) and \( l_1 = 2l_2 \), If the vibrational energies of both are same, then which of the following is correct?
View Solution
If 10% of a radioactive substance decays in every 5 years, then the percentage of the substance that will have decayed in 20 years, will be:
View Solution
Ball A moving at 12 m/s collides elastically with ball B, initially at rest as shown. If both balls have the same mass, then what is the final velocity of ball A?
View Solution
A stretched string of length 1 m fixed at both ends, when vibrated in one loop has a frequency of 200 Hz. It is now plucked at a point situated at 25 cm from one end. The stretched string would vibrate with a frequency of:
View Solution
A and B are at an angle of 60° with each other. Their resultant makes an angle of 45° with a. If \( b = 2 \) units, then \( a \) is:
View Solution
A beam of light (\( \lambda = 600 \, nm \)) from a distant source, falls on a single slit 1 mm wide and the resulting diffraction pattern is observed on a screen 2 m away. The distance between the first dark fringes on either side of the central bright fringe is:
View Solution
A liquid is poured into a vessel at rest with the hole in a wall closed by a valve. It is filled to height \( H \). The distance of the hole from the top surface is \( h \). What is the horizontal acceleration required to move the vessel so that the liquid does not come out when the valve is opened (given \( l = \) length of the base)?
View Solution
A glass prism ABC (refractive index 1.5), immersed in water (refractive index \( \frac{4}{3} \)). A ray of light is incident normally on face AB. If it is totally reflected at face AC, then
View Solution
Two trains A and B of length 400 m each are moving on two parallel tracks with a uniform speed of 72 km/h in the same direction, with A ahead of speed B. The driver of B decides to overtake A and accelerates by 1 m/s². If after 50s, the guard of B just brushes past the driver of A, what was the original distance between them?
View Solution
An ideal gas is taken through a cyclic thermodynamical process through four steps. The amounts of heat involved in these steps are \( Q_1 = 5960 \, J \), \( Q_2 = -5585 \, J \), \( Q_3 = -2980 \, J \) and \( Q_4 = 3645 \, J \), respectively. The value of \( W_4 \) is:
View Solution
A rocket is fired from the Earth towards the Sun. At what distance from the Earth's centre, the gravitational force on the rocket is zero? Mass of the Sun = \( 2 \times 10^{30} \, kg \) and mass of the Earth = \( 6 \times 10^{24} \, kg \).
View Solution
A block of mass m slides down with uniform speed on an inclined plane having inclination \( \theta \). If the coefficient of friction between the inclined plane and the block is \( \mu \), then the contact force between them is:
View Solution
A solid cylinder of mass 20 kg rotates about its axis with angular speed 100 rad/s. The radius of the cylinder is 0.25 m. What is the kinetic energy associated with the rotation of the cylinder? What is the magnitude of angular momentum of the cylinder about its axis?
View Solution
A body of density \( D \), and mass \( M \) is moving downward in glycerine of density \( D_z \). What is the viscous force acting on it?
View Solution
A stone of mass 0.25 kg tied to the end of a string is whirled round in a circle of radius 1.5 m with speed 40 rev/min in a horizontal plane. What is the tension in the string and what is the maximum speed with which the stone can be whirled around, if the string can withstand a maximum tension of 200 N?
View Solution
If \( N_0 \) be the number of nuclei present at time \( t = 0 \), then the number of undecayed nuclei, \( N \), present after \( n \) mean life is
View Solution
A U-shaped wire is dipped in a soap solution and removed. The thin soap film formed between the wire and light slider supports a weight of \( 1.5 \times 10^{-2} \, N \). The length of the slider is 30 cm. What is the surface tension of the film?
View Solution
An object stands 4 cm in front of a converging lens. If the lens has a focal distance of 1 cm, where is the image formed?
View Solution
The length of a magnet is large compared to its width and breadth. The time period of its oscillation in vibration magnetometer is 2 s. The magnet is cut along its length into three equal parts and three parts are then placed on each other with their like poles together. The time period of this combination will be:
View Solution
The image seen in a flat bathroom mirror is a
View Solution
If electrical force between two charges is 200 N and we increase 10% charge on one of the charges and decrease 10% charge on the other, then electrical force between them for the same distance becomes
View Solution
Electrons are accelerated through a potential difference \( V_e \), and protons are accelerated through a potential difference of 4 V. The de-Broglie wavelengths are \( \lambda_e \) and \( \lambda_p \) for electrons and protons, respectively. The ratio of \( \frac{\lambda_e}{\lambda_p} \) is given by (given \( m_e \) is mass of electrons and \( m_p \) is mass of protons):
View Solution
In hydrogen atom spectrum, frequency of \( 2.7 \times 10^{15} \, Hz \) of EM wave is emitted when transmission takes place from 2 to 1, the frequency emitted will be
View Solution
Given
I. Plane mirrors
II. Concave mirrors
III. Convex mirrors
Given the preceding choices, virtual images can be formed by:
View Solution
Consider the following reaction sequence of alkene. \[ CH_3CH = CH_3 \xrightarrow{O_3} A \xrightarrow{H_2O} B \]
What is the product B?
View Solution
Which of the following transition of an electron in H-atom will emit maximum energy?
View Solution
Which of the following compounds produces the most heat per mole of compound when reacted with oxygen?
View Solution
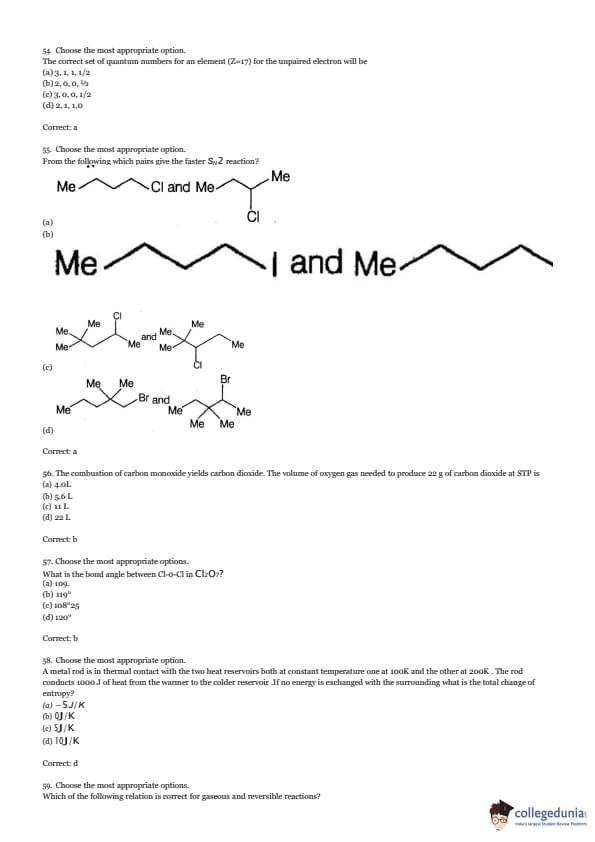
The correct set of quantum numbers for an element (Z=17) for the unpaired electron will be
View Solution
From the following which pairs give the faster SN2 reaction?
View Solution
The combustion of carbon monoxide yields carbon dioxide. The volume of oxygen gas needed to produce 22 g of carbon dioxide at STP is
View Solution
What is the bond angle between Cl-O-Cl in Cl\(_2\)O\(_7\)?
View Solution
A metal rod is in thermal contact with the two heat reservoirs both at constant temperature, one at 100K and the other at 200K. The rod conducts 1000 J of heat from the warmer to the colder reservoir. If no energy is exchanged with the surroundings, what is the total change of entropy?
View Solution
Which of the following relation is correct for gaseous and reversible reactions?
View Solution
What is the conjugate base of H\(_2\)SO\(_4\)?
View Solution
If the dipole moment of HBr is \( 2.60 \times 10^{-30} \, Cm \) and the interatomic spacing is 1.41 Å, then the percent ionic character of HBr is:
View Solution
In the redox reaction \[ MnO_4^- + C_2O_4^{2-} \to Mn^{2+} + CO_2 + H_2O \]
The correct coefficients of \( C_2O_4^{2-} \) and \( \text{H^+ \) \text{are
View Solution
In a sodium-sulphur battery, what is the half reaction for sodium in the spontaneous direction?
View Solution
Which of the following is not a true statement about ozone?
View Solution
Benzene and toluene combine to form an ideal solution. At 80°C, vapour pressure of pure benzene is 800 mmHg and the vapour pressure of pure toluene is 300 mmHg. If the vapour pressure of the solution is 400 mmHg, what are the mole fractions of benzene and toluene?
View Solution
Which of the following statements about halogens are correct?
View Solution
Compound A undergoes the following reaction to form B and C: \[ KNO_3 + KOH \to Black coloured solid (A) \xrightarrow{heated} Green coloured solid B + Pink solution + A (C decolorised by Fe^{2+}) \]
Identify A, B, and C respectively
View Solution
Terylene is a condensation polymer of ethylene glycol and
View Solution
When a sample of aluminium of unknown mass was subjected to 1.8 kJ of heat, the temperature of the aluminium sample increased from 26°C to 31°C. What was the mass of the sample?
(specific heat of Al = 0.90 J/g°C)
View Solution
When Z grams of calcium carbonate completely burnt in air gives 28g of a solid compound, the mass of calcium carbonate used will be:
View Solution
The correct increasing order of solubility of sulphates in water is:
View Solution
Sulphur(s) when react with HNO\(_3\) (conc.) Green mainly gives:
View Solution
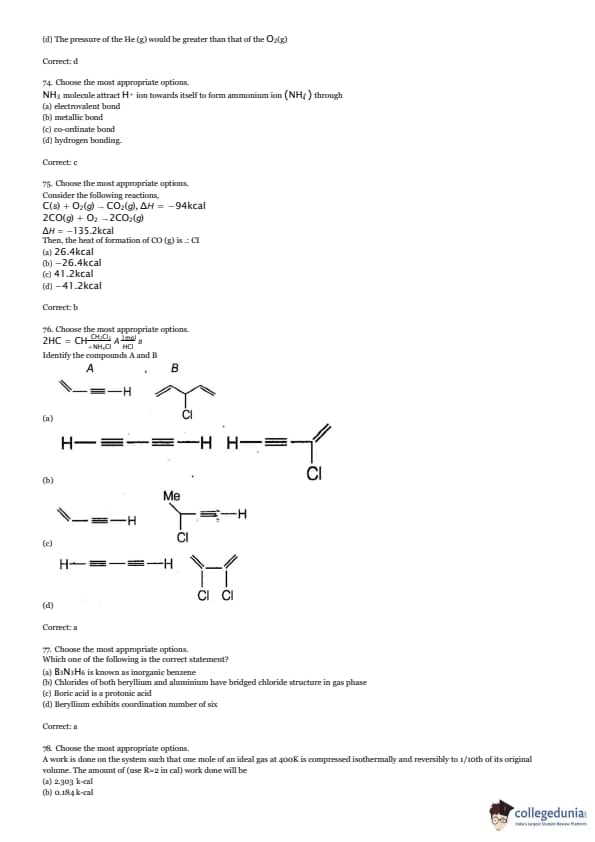
100 g of O2 and 100 g of He(g) are in separate containers of equal volume. Both gases are at 100°C. Which one of the following statements is true?
View Solution
NH_3 molecule attract H^+ ion towards itself to form ammonium ion (NH_4^+) through:
View Solution
Consider the following reactions,
C(s) + O2(g) → CO2(g), \Delta H = -94\text{ kcal
2CO(g) + O2 → 2CO2(g), \Delta H = -135.2\text{ kcalThen, the heat of formation of CO (g) is:
View Solution
2HC = CH_2\text{Cl_2 \xrightarrow{\text{A \text{NH_4\text{Cl \xrightarrow{\text{B \text{Identify the compounds A and B
View Solution
Which one of the following is the correct statement?
View Solution
A work is done on the system such that one mole of an ideal gas at 400K is compressed isothermally and reversibly to 1/10th of its original volume. The amount of (use R=2 cal) work done will be
View Solution
The atomic radius of Ne is
View Solution
The process involves smelting is
View Solution
Consider the following mechanism, \[ Cl + O_3 \rightarrow ClO + O_2 \] \[ ClO + O \rightarrow Cl + O_2 \]
In this mechanism, what is the catalyst?
View Solution
Which of the following is used as a food preservative?
View Solution
Silica is a network solid of silicon and oxygen atoms. The empirical formula for silica is SiO₂. In silica, to how many oxygen atoms is each silicon bonded?
View Solution
Bakelite is obtained by reaction of phenol with
View Solution
Curve X on the graph below shows the volume of oxygen formed during the catalytic decomposition of a 1.0 mol dm\(^{-3}\) solution of hydrogen peroxide.
\includegraphics{ig13.jpeg % Include your image here
Which change would produce the curve Y?
View Solution
Rate of dehydration of alcohols follows the order
View Solution
As temperature is increased, the equilibrium of a gaseous reaction will always
View Solution
The ozone layer forms naturally by
View Solution
Which of the following statement is applicable for the Tyndall effect?
View Solution
A catalyst will change all of the following except
View Solution
On reduction of glycolic acid with HI, the product formed is
View Solution
The metallic sodium dissolved in liquid ammonia to form a deep blue colour solution. The deep blue colour is due to the formation of
View Solution
Which of the following statements is not correct?
View Solution
Which of the following flux is used to remove acidic impurities in metallurgical processes?
View Solution
A cell has been set up as shown in the following diagram and E\(^0\) has been measured as 1.00V at 25°C. Calculate \(\Delta\)G\(^0\) for the reaction.
View Solution
Select the incorrect statement.
View Solution
Which of the following is the correct Lewis structure for the ionic compound Ca(ClO2)2?
View Solution
For the reactions
C + O2 \rightarrow CO2; \, \Delta H = -393 \, \text{kJ
2Zn + O2 \rightarrow 2ZnO, \, \Delta H = -412 \, \text{kJ
\text{the correct statement is:
View Solution
The decreasing order of reactivity towards electrophilic addition of the following is:
I. CH = CH
II. CH_2 = CH_2
III. H_2C = CH - Cl
View Solution
An aqueous solution of 2% (wt/wt) non-volatile solute exerts a pressure of 1.004 bar at the boiling point of the solvent. What is the molecular mass of the solute?
View Solution
Consider line segments of lengths 1, 2, 3, \dots, 10, what is the number of triangles that can be formed from them?
View Solution
The value of \[ \lim_{x \to 0} \int_0^x \sec^2 t \, dt \]
is:
View Solution
How many paths are there from the point A to the point B in the figure below, if no point in a path is to be traversed more than once?
View Solution
The number of real roots of \[ (6 - x)^4 + (8 - x)^4 = 16 \]
View Solution
The expression \[ \left| (a + 1)^2 \, (b + 1)^2 \, (c + 1)^2 \right| \]
is equal to
View Solution
The function \( f:[0,3] \to [1,29] \) defined by \[ f(x) = 2x^3 - 15x^2 + 36x + 1 \]
is
View Solution
For all \( n \in \mathbb{N}, 72n - 48n - 1 \) is divisible by
View Solution
The sum \[ \sum_{k=0}^5 \left( 5C_k \right)^2 \]
is equal to
View Solution
If \[ f(x) = \begin{cases} \frac{1 - \sin x}{(n - 2x)^2} & if \quad x \neq \frac{\pi}{2}
\log (\sin x) \cdot \log \left( 1 + \frac{\pi}{4x + x^2} \right) & if \quad x = \frac{\pi}{2} \end{cases} \]
is continuous at \( x = \frac{\pi}{2} \), then \( k \) is equal to
View Solution
The length of the axes of the conic \[ 9x^2 + 4y^2 - 6x + 4y + 1 = 0 \]
are
View Solution
A differentiable function \( f(x) \) has a relative minimum at \( x = 0 \), then the function \( y = f(x) + ax + b \) has a relative minimum at \( x = 0 \) for
View Solution
The maximum number of points into which 4 circles and 4 straight lines intersect, is
View Solution
The solution of the differential equation \[ (x^2 - yx^2) \frac{dy}{dx} + y^2 + xy^2 = 0 \]
is
View Solution
Given that \( \alpha_1, \alpha_2, \alpha_3 \) are the roots of \( 3x^3 - x^2 - 10x + 8 = 0 \), then the value of \( \alpha_1^2 + \alpha_2^2 + \alpha_3^2 \) is
View Solution
The area bounded by the curves \( y = \cos x \) and \( y = \sin x \) between the ordinates \( x = 0 \) and \( x = \frac{3\pi}{2} \) is
View Solution
The integral \[ \int_0^{\frac{\pi}{2}} \frac{\sqrt{\sin x + \cos x}}{\sin x + \cos x} \, dx \]
is equal to
View Solution
The value of the integral \[ \int_0^0 9 [x - 2\lfloor x \rfloor] \, dx \]
where \( [.] \) denotes the greatest integer function, is
View Solution
The value of \[ \int_0^{\frac{\pi}{2}} \sin^7 \theta \cos^4 \theta \, d\theta \]
is
View Solution
The number of solutions of the equation \[ 3 \sin^2 x - 7 \sin x + 2 = 0 \]
in the interval \( [0, 5\pi] \) is
View Solution
The inverse of matrix \[ \begin{pmatrix} 0 & 1 & -1
4 & -3 & 4
3 & -3 & 4 \end{pmatrix} \]
is
View Solution
If \[ a = \gamma \hat{i} + \delta \hat{j} + 2\zeta \hat{k}, \, b = \alpha \hat{i} + \beta \hat{j} + \gamma \hat{k}, \, r \times a = b \times a \, r \times b = a \times b \]
then a unit vector in the direction of \( r \) is
View Solution
The solution of the differential equation \[ \frac{d^2y}{dx^2} + 3y = -2x \]
is
View Solution
From the bottom of a pole of height h, the angle of elevation of the top of a tower is \( \alpha \). The pole subtends an angle \( \beta \) at the top of the tower. The height of the tower is
View Solution
If the numbers \( a_1, a_2, \ldots, a_n \) are different from zero and form an arithmetic progression, then \[ \frac{1}{a_1} + \frac{1}{a_2} + \frac{1}{a_3} + \dots + \frac{1}{a_n} \]
is equal to
View Solution
The equation of a straight line passing through the point of intersection of \( x - y + 1 = 0 \) and \( 3x + y - 5 = 0 \) and perpendicular to one of them, is:
View Solution
The number of integral values of \( \lambda \) for which the equation \[ x^2 + y^2 - 2\lambda x + 2\lambda y + 14 = 0 \]
represents a circle whose radius cannot exceed 6 is:
View Solution
Let \( x_1 \) and \( x_2 \) be the roots of the equation \[ ax^2 + bx + c = 0 \quad (ac \neq 0) \]
Find the value of \[ \frac{1}{x_1} + \frac{1}{x_2} \]
View Solution
A line makes the same angle \(\theta\) with each of the X and Z-axes. If the angle \(\beta\) which it makes with Y-axis is such that \(\sin^2 \beta = 3 \sin^2 \theta\), then \(\cos^2 \theta\) equals
View Solution
The mid-point of the chord \(2x + y - 4 = 0\) of the parabola \(y^2 = 4x\) is
View Solution
Gas is being pumped into a spherical balloon. Then, the rate at of \(30 \, ft^3/min\) which the radius increases when it reaches the value 15 ft, is
View Solution
If \(n\) is an integer, compute the value of the fraction
\[ \frac{(1 + \theta)^n}{(1 - \theta)^{n-2}} \]
View Solution
If the tangent at (1,1) on \( y^2 = x(2 - x^2) \) meets the curve again at P, then P is:
View Solution
If \( |a| < 1 \) and \( |b| < 1 \), then the sum of the series \[ a(a + b) + a^2(a^2 + b^2) + a^3(a^3 + b^3) + \cdots \]
View Solution
Three points are chosen randomly and independently on a circle. What is the probability that all three pairwise distances between the points are less than the radius of the circle?
View Solution
Choose the most appropriate option.
If \( A \) is a square matrix such that \( A^2 = A \) and \( B = I \), then \( AB + BA + I - (I - A)^2 \) is equal to:
View Solution
Calculate: \[ \left| \begin{matrix} x & y & x + y
y & x + y & x
x + y & x & y
\end{matrix} \right| \]
View Solution
Let \( f(x) = (x^3 + 2)^{30} \). If \( f^{(n)}(x) \) is a polynomial of degree \( 20 \), where \( f^{(n)}(x) \) denotes the \( n + h \)-order derivative of \( f(x) \), then the value of \( n \) is:
View Solution
The set of values of \( x \) satisfying the system of inequalities \( 5x + 2 < 3x + 8 \) and \( \frac{x^2}{x-1} < 4 \) is:
View Solution
Choose the most appropriate option.
The value of \(\lim_{x \to a \frac{\log x - 1}{x - a}\) is equal to
View Solution
Choose the most appropriate options.
\text{If \( |z^2 - 1| = |z|^2 + 1 \), \text{then \( z \) lies on a
View Solution
Choose the most appropriate options.
\text{If \( f(x) = [x \sin n\pi x] \), \text{then which of the following is incorrect?
View Solution
Choose the most appropriate option.
\text{Find the distance from the point A (2, 3, -1) to the given straight line. \[ x = 3t + 5, \quad y = 2t, \quad z = -2t - 25 \]
View Solution
Choose the most appropriate options.
The degree of the differential equation \[ x = 1 + \frac{dy}{dx} + \frac{1}{2!} \left( \frac{d^2y}{dx^2} \right) + \frac{1}{3!} \left( \frac{d^3y}{dx^3} \right) + \dots \]
View Solution
Choose the most appropriate option.
On the sphere \( (x - 1)^2 + (y + 2)^2 + (z - 3)^2 = 25 \), find the point \( M_0 \) to the plane \( 3x - 4z + 19 \).
View Solution
Choose the most appropriate options.
The limit \[ \lim_{x \to 0} \frac{1 - \cos 2x}{x \tan 4x} \]
View Solution
Choose the most appropriate options.
If \( A(2,3) \) and \( B(-2,1) \) are two vertices of a triangle and the third vertex moves on the line \( 2x + 3y = 9 \), then the locus of the centroid of the new set of observations will be the triangle is
View Solution
Choose the most appropriate option. \[ \lim_{x \to 0} \frac{\sin 2x}{\sin x} \]
View Solution
Choose the most appropriate options.
Let \( f(x) = ax^3 + 5x^2 - bx + 1 \). If when divided by \( x - 1 \) it leaves a remainder of 5, and \( f(x) \) is divisible by \( 3x - 1 \), then
View Solution
Choose the most appropriate options.
If the SD of a set of observations is 8 and each observation is divided by -2, then the SD of the new set of observation will be:
View Solution
Choose the most appropriate option. \[ \lim_{x \to 1} \sin(x - 1) \cdot \tan\left( \frac{\pi}{x} \right) \]
View Solution
















































































Comments