Jasmine Grover Content Strategy Manager
Content Strategy Manager | Updated On - Sep 26, 2025
A colorimeter is a light-sensitive lab instrument that is utilized to determine the concentration of a known solute in a solution. It is used to measure the absorbance and transmittance of light that passes through a liquid.
- A colorimeter gives the measurement of absorbance and transmittance percentage in a solution.
- The wavelength used for performing the colorimetric experiment is 620 nm, as the scattering of light is minimum at this wavelength.
- The percentage of light passing through a solution is considered the transmittance percentage.
- Absorbance determines the amount of light absorbed by the solution.
- The use of a colorimeter is seen in manufacturing industries, laboratories, paints, testing water quality, and determining hemoglobin concentration in blood.
Colorimeters are sometimes called spectrophotometers, but they are actually two different instruments. Although colorimeters and spectrophotometers work similarly, spectrophotometers provide more complex and advanced color data.
Related Links
Key Terms: Colorimeter Principle, Beer-Lambert’s Law, Solution concentration, Optical Density, Colorimeter, Wavelength
Key Summary
In this article, we will discuss the colorimeter, its uses, working and advantages, and disadvantages in detail.
- The principle of the Colorimeter is that coloured compounds can absorb a certain wavelength of light when monochromatic light is passed through them.
- The working of a colorimeter and spectrophotometer is based on the concept of Beer-Lambert’s law.
- Calibration is done once through software to avoid calculation errors, and then the cuvette used in the colorimetry experiment is cleaned with distilled water.
What is a Colorimeter?
Colorimeters are used to detect colour and determine a solution’s concentration. When a wavelength is passed through a sample, some of the light gets absorbed and some passes through. The passing wavelengths of light are detected.

Colorimeter
Ques: What is the use of a colorimeter in laboratories?
Ans: The measurement of the absorbance of a specific wavelength of light in a solution is done by a colorimeter. This further helps in determining the concentration of solutes such as proteins, glucose, or enzymes.
Ques: What is the basic principle behind a colorimeter?
Ans: An instrument called a colorimeter works on the principle of Beer & Lambert’s law. The law states that the absorbance of light by a solution is directly proportional to the concentration of the solute present in it.
Applications of the Colorimeter:
- A colorimeter is most commonly used to determine the concentration of a coloured compound by measuring the absorbance or optical density.
- In the case of colourless compounds, a suitable reagent is introduced, which, when mixed, would result in a coloured compound. This is then measured in the colorimeter against the known values of the standard solution.
- The course of a reaction can be determined in a colorimeter by measuring the rate of formation and disappearance of the light-absorbing compound.
- A colorimeter can also act in the reverse process, identifying a compound by measuring its absorption index.
Read More:
| Learn more about Colorimetry | |
|---|---|
| Brewster's Law | Coherent and Incoherent Addition of Waves |
| Wave Theory of Light | Transmission, Absorption, and Reflection of Light |
Principle of Colorimeter
The principle of Colorimeter is based on the photometric technique that states when an incident light of intensity (I0) passes through a solution, then
- Part of the incident light is reflected (Ir)
- Part of the incident light is transmitted (It)
- Part of the incident light is absorbed (Ia)
Therefore,
| I0 = Ir + It + Ia |
|---|
Here, the value of reflected light (Ir) is eliminated as I0, and the It values are enough to calculate Ia. Values for the amount of light absorbed and transmitted are measured by keeping the incident radiation (Ir) constant. The principle of the colorimeter is based on two fundamental laws of photometry that establish the relationship between the amount of light absorbed and the concentration of the substance.
Ques. What are the different types of colorimeters?
Ans. Two types of colorimeters are the spectrophotometer and the tristimulus colorimeter.
- Tristimulus colorimeters: These are mainly used in quality control and are reliable for evaluating colour differences and colour tolerance.
- Spectrophotometers: They analyze the light reflected or transmitted by a sample at each wavelength in the visible spectrum, compared to that from a reference sample.
Ques: How do Beer & Lambert’s law help in understanding the principle of colorimetry?
Ans: According to this law, which was formulated by two biologists, Beer and Lambert (commonly called Beer-Lambert’s law), the decrease in intensity can also be measured.
- By passing light of a specific wavelength through a colored solution, the decrease in intensity is measured.
- This decrease helps in quantifying the solute concentration in unknown samples.
Beer-Lambert’s Law
Beer’s Law states that the amount of light absorbed is directly proportional to the concentration of the solute in the solution.
| Log10 I0/ It = asc |
|---|
Where,
- as → Absorbency Index
- c → Concentration of solution
Lambert’s Law states that the amount of light absorbed is directly proportional to the length and thickness of the solution under analysis.
| Log10 I0/ It = asb |
|---|
Where,
- A → Test of Absorbance
- → Absorbance of standard solution
- b → length or thickness of the solution
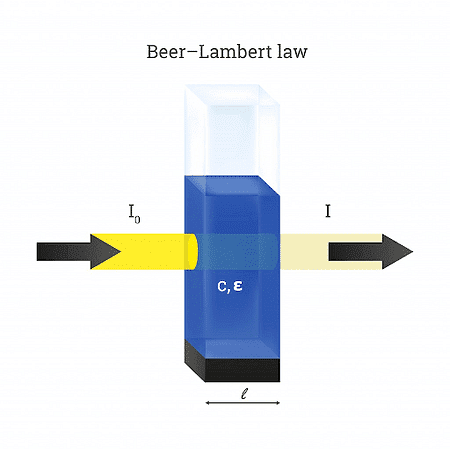
Beer-Lambert’s Law
The combined mathematical expression of the Beer-Lambert Law is:Log10 I0/ It = asbc If b is kept constant by taking a cuvette or a standard cell, then: Log10 I0/ It = asc Where the Absorption index is given by: as = A/cl |
|---|
In the combined mathematical expression of Beer-Lambert’s Law,
- A → Absorbance or optical density of the solution
- c → Concentration of the absorbing material (gm/lit)
- l → distance travelled by light in solution (cm)
In simple terms, the combined principle of Beer-Lambert’s law states that the amount of light absorbed by a colour solution is directly proportional to the concentration of the solution and the length of the light path through the solution, A ∝ cl.
| Thus, A = ∈cl |
|---|
Where,
∈ → Absorption Coefficient
Ques: State the equation of Beer-Lambert’s Law in a colorimeter?
Ans: The equation of Beer-Lambert’s law utilized in understanding the mechanism of the colorimeter is given as:
A =ε × c × l
Where,
ε = molar absorptivity,
c = concentration, and
I = path length of light.
Ques. What is Colorimetry Absorbance?
Ans. Colorimetry absorbance is the unit of measurement for the amount of light that passes through the given solution at a specific wavelength. It is the ratio between the amount of light absorbed to the total amount of light available at that wavelength.
Components of Colorimeter
The main parts that make up the colorimeter are:
- Source of Light: Tungsten filament is commonly used as a light source in colorimeters.
- Monochromator: It is used to split the light into different wavelengths and select the particular wavelength under observation.
- Sample Holder: This is where the cuvettes or test tubes containing the colour sample solution are placed. These are made of glass at visible wavelengths.
- Photo Detector System: This system produces an electric signal when light falls into it, which is in turn reflected as a reading in the galvanometer.
- Measuring Device: The galvanometer is used as the measuring device, which converts the electrical signals into readings that correspond to the intensity of light.
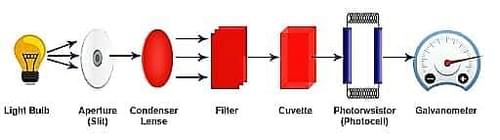
Components of the Colorimeter
Ques. What are the types of light sources that are normally used in colorimeters and spectrophotometers?
Ans. The types of light sources used are:
- For the Colorimeter, LED light sources of fixed wavelength are used.
- For Spectrophotometers: Tungsten, Xenon, Deuterium lamps consisting of a wide range of wavelengths are used.
Working Of the Colorimeter
The working mechanism of a Colorimeter is explained below in step-by-step:
- The Colorimeter has to be calibrated first by using the standard solutions of the known concentrations of the solute that is to be determined in the test solution.
- The standard solutions are poured into the cuvettes, which are then placed in the sample holder.
- A beam of light of a certain wavelength specific to the assay is directed towards the test solution.
- Before it reaches the test solution, it passes through a series of lenses and filters. The lens helps in the accurate navigation of the beam of light.
- The filters split the incoming light into different wavelengths and allow the required wavelength to reach the cuvette containing the test solution.
- The monochromatic light (light of one wavelength) reaches the test solutions, and some of the light gets reflected, some gets absorbed, and the remaining would passes through the test solution and falls onto the photodetector.
- The photodetector sends the pulses to the galvanometer. The galvanometer reads the electrical signals from the detector and displays them in digital form.
- The reading corresponds to the absorbance or the optical density of the test solution.
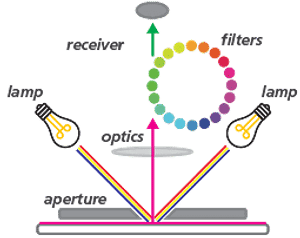
Working of the Colorimeter
| We know that, A = ∈cl For standard and test solutions, ∈ and l are constant Therefore, AT = CT …(i) As = Cs …(ii) -multiplying (i) and (ii) AT x Cs = CT x As CT = (AT / As) x Cs Where,
|
|---|
Ques: How does a colorimeter work step by step?
Ans: Light passes — Selected wavelength filter — Passes through sample — Detector measures transmitted light — Converted into absorbance/concentration data.
Ques: What is the role of calibration in a colorimeter's working?
Ans: Calibration with standard solutions ensures accurate absorbance readings and corrects for any errors in the device/system.
Uses of Colorimeter
The uses of a Colorimeter are as follows:
- Colorimeter is widely used in the medical industry to estimate biochemical samples such as blood, urine, cerebral spinal fluid, plasma, serum, etc.
- They are used to analyse the colour contrast and brightness in mobile, computer, and television screens to provide users with the best viewing experience.
- It also finds its application in the paints and textile industries. A colorimeter is used in the food and food processing industry.
- It is used in the printing industry to measure the quality of print paper and printing ink.
- They are also used to test the water quality and screen for the identification of chemical substances such as chlorine, fluorine, cyanide, iron, molybdenum, etc.
- They are used in jewellery to measure diamond quality.
- A colorimeter is used to measure the concentration of haemoglobin in blood samples.
- It helps to monitor the nutrient concentration in the soil for plant growth.
- A colorimeter is also used in the pharmaceutical industry to identify substandard products and drugs.
Also check:
| Important Chemistry Practicals | ||
|---|---|---|
| Rate of Reaction | Emulsifying Agents in Stabilising Emulsions of Oils | Transition Elements Oxidation States |
Ques: What are the common applications of a colorimeter in biology?
Ans: Used in clinical labs for blood glucose tests, enzyme assays, and protein concentration determination.
Ques: How is a colorimeter used in industry?
Ans: Applied in the food, textile, and paint industries for color matching, quality control, and chemical concentration analysis.
Advantages and Disadvantages of Colorimeter
The advantages and disadvantages of using a colorimeter are specified below:
Advantages of the Colorimeter
Disadvantages of the Colorimeter
|
|---|
Read More:
| Learn more about Wave Optics | ||
|---|---|---|
| Wave Optics | Huygens Principle | Polarisation |
| Diffraction | Interference of Light Waves and Young’s Experiment | Difference between diffraction and interference |
| Destructive Interference | Relation between phase difference and path difference | Resolving power of microscopes and telescopes |
Colorimeter Price Comparison Table
The price of a colorimeter varies depending on the model, purpose, and country. Here, we've provided the price range for colorimeters (basic and advanced) for India, the UK, the US, and other countries:
| Country | Average Price (Basic Model) | Average Price (Advanced Model) |
|---|---|---|
| India | Rs. 5,000 – Rs. 15,000 | Rs. 20,000 – Rs. 60,000 |
| USA | $100 – $200 (Rs. 8,000 – Rs. 16,000) | $500 – 1,200 (Rs. 40,000 – Rs. 96,000) |
| UK | £ 90 – £ 180 (Rs. 9,000 –Rs. 18,000) | £400 – £ 950 (Rs. 40,000 – Rs. 95,000) |
| Other Countries (Avg.) | Rs. 10,000 – Rs. 20,000 | Rs. 50,000 – Rs. 1,00,000 |
Things To Remember
- A colorimeter is used to measure the intensity of a coloured compound.
- It is operative in the visible range of the electromagnetic spectrum.
- A colorimeter works on the principle of Beer-Lambert’s law.
- It should be calibrated by using a standard solution of known concentration.
- It has a wide variety of applications in the field of medicine, textiles, printing, food processing, agriculture, etc.
- They are used in jewellery to measure diamond quality.
Sample Questions
Ques. What are the steps involved in using a colorimeter?
Ans. The steps involved in using a colorimeter are given below:
| Step | Process | Purpose |
|---|---|---|
| Step 1 | Prepare standard and test solutions | To compare an unknown concentration with a known |
| Step 2 | Switch on and calibrate the colorimeter | To set zero absorbance with a blank solution |
| Step 3 | Select an appropriate filter | To allow light of the desired wavelength |
| Step 4 | Place a blank solution in the cuvette | For baseline correction |
| Step 5 | Measure the absorbance of standard solutions | To plot a standard calibration curve |
| Step 6 | Measure the absorbance of the test solution | To find its concentration |
| Step 7 | Compare the test absorbance with the calibration curve | To determine the unknown concentration |
Ques. How do colorimeter and spectrophotometers differs?
Ans. The differences between a colorimeter and a spectrophotometer are based on the following parameters:
| Feature | Colorimeter | Spectrophotometer |
|---|---|---|
| Wavelength Range | Uses fixed filters (limited range) | Wide wavelength range (UV, Visible, IR) |
| Accuracy | Good for colored solutions | Higher accuracy, even for colorless solutions |
| Cost | More affordable | More expensive |
Ques. State the principles of the colorimeter. (2 marks)
Ans. The Colorimeter works on the principle of Beer-Lambert’s law.
- Beer’s Law: This law states that the amount of light absorbed is directly proportional to the concentration of the solute in the solution.
- Lambert’s Law: This law states that the amount of light absorbed is directly proportional to the length and thickness of the solution under analysis.
Ques. Mention the disadvantages of the Colorimeter. (2 marks)
Ans. The disadvantages of a colorimeter are as follows:
- It becomes a tedious process to identify the concentration of colourless compounds.
- Since it measures the absorbance of wavelength only in the visible spectrum of light (400nm to 700nm), it does not work in the ultraviolet and infrared spectra.
- It is not possible to set a specific wavelength; rather, a range of the spectrum has to be set to measure the absorbance.
Ques. Diagrammatically represent the essential parts of a colorimeter. (2 marks)
Ans. The essential parts of a colorimeter are the light source, monochromator, cuvette holder, photodetector system, and galvanometer.
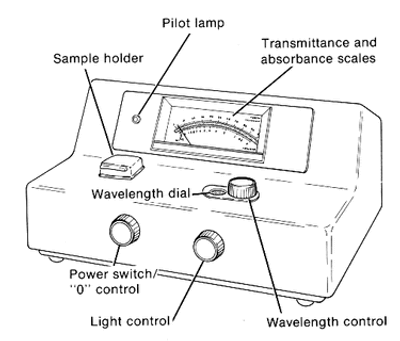
Colorimeter Diagram
Ques. Mention any 5 uses of the colorimeter. (3 marks)
Ans. A colorimeter can be used in the following ways:
- It is widely used in the medical industry to estimate biochemical samples such as blood, urine, cerebrospinal fluid, plasma, serum, etc.
- They are also used to test the water quality and screen for the identification of chemical substances such as chlorine, fluorine, cyanide, iron, molybdenum, etc.
- It is used in the printing industry to measure the quality of print paper and printing ink.
- It helps monitor the nutrient concentration in the soil for plant growth.
- It is also used in the pharmaceutical industry to identify substandard products and drugs.
Ques.What is the working range of the colorimeter? (2 marks)
Ans. The colorimeter is operative in the visible range of the electromagnetic spectrum. I.e, It can operate in wavelengths ranging from 400nm to 700nm. However, for a standard colorimeter, the ranges are predefined and the users can select from wavelengths: 430nm, 470nm, 565nm, 635nm.
Ques. What is the difference between a colorimeter and a spectrophotometer? (2 marks)
Ans. Colorimeters are usually portable and use LED light sources and colour filters. As a result, they operate at fixed wavelengths and can only accommodate tests that incorporate those wavelengths.
Spectrophotometers are usually benchtop instruments and use light sources that can produce a range of wavelengths. Spectrophotometers also use monochromators to select the desired wavelength. As a result, spectrophotometers can be used for a broad range of tests.
Ques. Mention some of the advantages of the Colorimeter. (2 marks)
Ans. Some of the advantages of a colorimeter are:
- It is a cheap and efficient method of quality analysis.
- Portable colorimeters are available, which makes them convenient to use.
- Quantitative analysis of coloured compounds can be easily done.
Ques. In which range is the colorimeter used? (2 marks)
Ans. Colorimeters are used in the range of 400nm to 800nm. To maximize accuracy, the colorimeter has changeable optical filters to pick the wavelength the solute collects the most. The wavelength range is typically 400 to 700 nm.
Ques. Which of the following is not an application of a colorimeter? (1 mark)
(i) Paints
(ii) Inks
(iii) Composition Detection
(iv) Cosmetics
Ans. Composition Detection.
Colorimeters are used to analyse the colors of different wavelengths in the visible range of the electromagnetic spectrum. Thus, it is not used to detect composition.
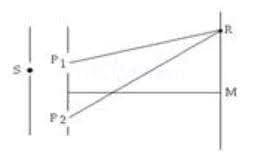
Ques.A slit S is illuminated by a monochromatic source of light to give two coherent sources, P1 and P2. These are the bright and dark bands on a screen. At a point R, on the screen, there is a dark fringe. What relation must exist between the lengths P1R and P2R? (3 marks)
Ans. There will be a dark fringe at point R when the path difference = P2R – P1R..
When λ is the wavelength of light and n = 0,1,2,3…
Que s.In Young’s double slit experiment, how does the fringe width change when:
(a) Light of a smaller frequency is used.
(b) Distance between the slits is decreased? (3 marks)
Ans.
(a) If light of a smaller frequency and a higher wavelength is used, the fringe width will increase.
β=Dλd
(b) If the distance between the slits is decreased, the fringe width will increase.
βα1d
Ques. Write the differences between inference and diffraction. (2 marks)
Ans.
| Interference | Diffraction |
|---|---|
| It is the superposition of light coming from two coherent sources | It is the superposition of the waves coming from different parts of the same wave front. |
| All bright fringes are of equal intensity. | The intensity of bright fringes decreases with increasing distance from the central bright fringes. |
Ques.Can white light produce interference? What is its nature? (2 marks)
Ans. White light produces interference. Different colour present in the white light interference pattern overlaps the central bright fringe for all the colours at the position. Thus, its colour is white. The white central bright fringe is surrounded by a few coloured rings.
Que s.(a) The refractive index of glass is 1.5. What is the speed of light in glass? The speed of light in a vacuum is 3x108 ms-1
(b) Are the speed of light in glass and its colour independent of each other? If not, does red or violet travel slower in a glass prism? (3 marks)
Ans.
(a) Refractive index of glass
μ = 1.5
Speed of light = 3x108 ms-1
We know that,
v=cλ
3x108/ 1.5 = 2x108 ms-1
(b) The speed of light in glass is not independent of the colour of light. The refractive index of a violet component of white light is greater than the refractive index of a red component. Hence, the speed of violet light is less than the speed of red light in glass. Hence, violet light travels more slowly than red light in a glass prism.
Ques. For sound waves, the Doppler formula for frequency shift differs slightly between the two situations:
(i) source at rest; observer moving, and
(ii) source moving; observer at rest.
The exact Doppler formulas for the case of light waves in a vacuum are, however, strictly identical for these situations. Explain why this should be so. Would you expect the formulas to be strictly identical for the two situations in case of light travelling in a medium? (3 marks)
Ans. No Sound waves need a medium to propagate. The two given situations are not scientifically identical because the motion of an observer relative to a medium is different in the two situations. Hence, the Doppler formulas for the two situations cannot be the same. In the case of light waves, they can travel in a vacuum. In a vacuum, the above two cases are identical because the speed of light is independent of the motion of the observer and the motion of the source. When light travels in a medium, the above two cases are not identical because the speed of light depends on the wavelength of the medium.
Ques.State the conditions that must be satisfied for two light sources to be coherent. (2 marks)
Ans. For two light sources to be coherent, they must:
(1) Emit waves continuously of the same wavelengths.
(2) The phase difference between the waves must be zero or constant.
Ques. A light wave enters from the air into the glass. How will the following be affected? (2 marks)
(i) Frequency of the wave
(ii) Energy of the wave
Ans. When light travels from a rarer medium to a denser medium, the following changes occur.
(1) Frequency of the wave remains the same.
(2) The light energy of the wave will be lower in the glass as part of the light is reflected to the atmosphere.



Comments