The application form for the Manipal Entrance Test (MET) 2026 is now open online at manipal.edu, with a deadline of March 15, 2026, for the first phase. The exam will be conducted in two phases in April and May and will be held online.
-
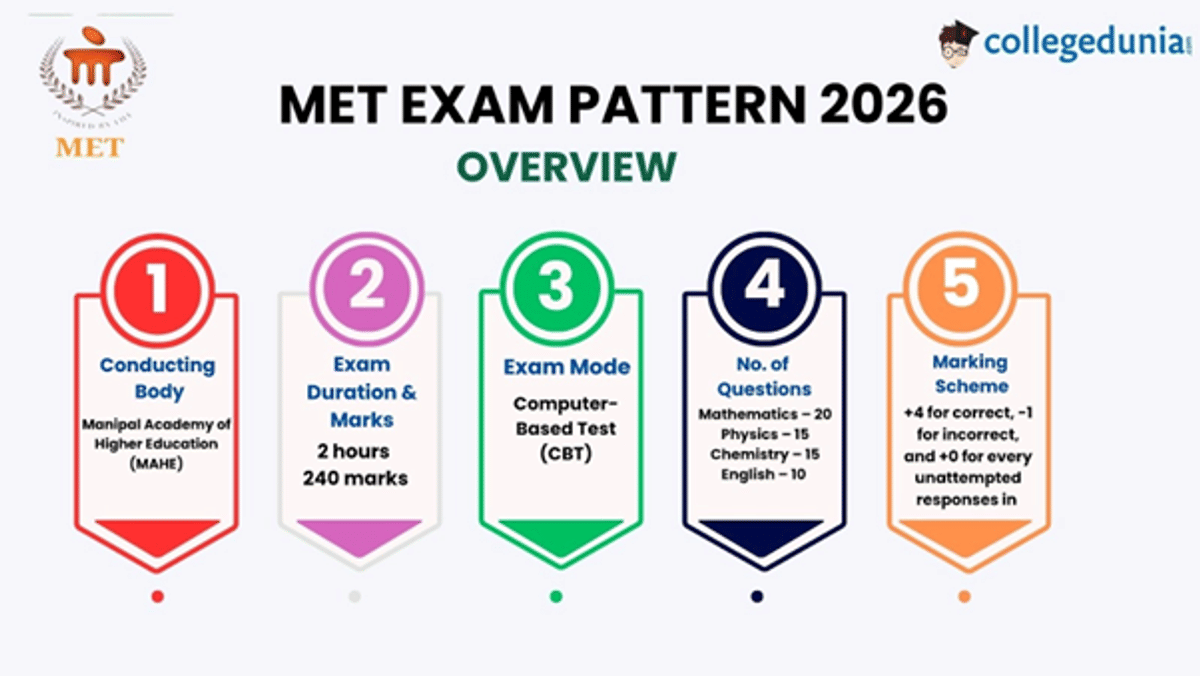
The MET exam pattern 2026 is online and computer-based. The MET exam is conducted for 2 hours, and you are supposed to answer a total of 60 questions.
- The MET exam pattern includes Physics and Chemistry question papers, which consist of 10 MCQs and 5 NATs.
- MET 2026 Mathematics question paper includes 15 MCQs and 5 NATs, and the English paper consists of only 10 MCQs.
You will be marked with +4 for correct, -1 for incorrect, and +0 for every unattempted response in the MET exam pattern 2026. Students' marks for the MET 2026 exam are calculated out of a total of 240.
Quick Links
Key Summary
In this article, we have discussed the MET Exam Pattern for 2026, including the marking scheme, syllabus, pattern, weightage, and preparation tips.
- The MET exam pattern 2026 is online and computer-based. The MET exam is conducted for 2 hours, and you are supposed to answer a total of 60 questions.
- The MET exam pattern includes Physics and Chemistry question papers that consist of 10 MCQs and 5 NATs.
- MET 2026 Mathematics question paper includes 15 MCQs and 5 NATs, and the English paper consists of only 10 MCQs.
- You will be marked with +4 for correct, -1 for incorrect, and +0 for every unattempted response in the MET 2026 exam. Students' marks for the MET 2026 exam are calculated out of a total of 240.
What is the MET Exam Pattern 2026?
The MET exam pattern 2026 is online and computer-based. The MET exam is conducted for 2 hours, and you are supposed to answer a total of 60 questions.
- The MET exam pattern includes Physics and Chemistry question papers that consist of 10 MCQs and 5 NATs.
- MET 2026 Mathematics question paper includes 15 MCQs and 5 NATs, and the English paper consists of only 10 MCQs.
- You will be marked with +4 for correct, -1 for incorrect, and +0 for every unattempted response in the MET exam pattern 2026. Students' marks for the MET 2026 exam are calculated out of a total of 240.
MET Exam Pattern 2026: Overview
| Particulars | Details |
|---|---|
| Conducting Body | Manipal Academy of Higher Education (MAHE) |
| Mode of Exam | Computer-Based Test (Online) |
| Duration | 2 Hours (120 Minutes) |
| Type of Questions | Multiple Choice Questions (MCQs) & Numerical Answer Type (NAT) |
| Sections & Questions | Mathematics – 15 MCQs + 5 NATsPhysics – 10 MCQs + 5 NATsChemistry – 10 MCQs + 5 NATsEnglish – 10 MCQs |
| Total Marks | 240 |
Ques. How should I manage time in MET 2026?
Ans. Since the exam has 60 questions in 120 minutes, candidates have approximately 2 minutes per question. A recommended strategy is:
- Attempt MCQs first, as they carry the same weight and are quicker to solve.
- Then focus on NAT questions, which may require longer calculations.
- Leave sufficient time for reviewing answers, especially for NAT questions, to avoid negative marking.
Ques. What types of questions are included in the MET exam 2026?
Ans. The MET Exam features two types of questions. The table below shows the types of questions:
| Question Type | Description | Options / Answer Format |
|---|---|---|
| Multiple Choice Questions (MCQs) | You must select the correct answer from the given choices. | 4 options, 1 correct answer |
| Numerical Answer Type (NAT) | You must calculate and enter the numerical value as the answer. | Enter numeric value (no options) |
MET Exam Pattern 2026: Marking Scheme
The marking scheme for the examination is different for various programs. It is given below:
- Marking Scheme for ME and M.Tech courses
- Marking Scheme for all other courses
- The major thing to notice about the marking scheme of all other courses under MET is that there is no negative marking.
- This simply means that the final scores will depend on the number of questions answered correctly, as incorrect answers will not lead to any deduction of marks.
| Type of Situation | Impact / Marks Awarded |
|---|---|
| Correct Answer (MCQ) | +4 marks |
| Incorrect Answer (MCQ) | −1 mark |
| No Answer (MCQ) | 0 marks |
| NAT Question | • +4 marks for correct answer• 0 marks for unattempted questions• 0 marks for wrong answers |
MET Exam Pattern 2026: Division of Questions
The MET 2026 question paper will consist of both multiple-choice and numerical answer-type questions. You can check the detailed structure of the MET question paper from the table given below.
| Sections | Type of Questions | Total Number of Questions |
|---|---|---|
| Physics | Multiple Choice Questions (MCQ) | 10 |
| Numerical Answer Type (NAT) Questions | 5 | |
| Chemistry | Multiple Choice Questions (MCQ) | 10 |
| Numerical Answer Type (NAT) Questions | 5 | |
| Mathematics | Multiple Choice Questions (MCQ) | 15 |
| Numerical Answer Type (NAT) Questions | 5 | |
| English | Multiple Choice Questions (MCQ) | 10 |

Check:
Ques. What is the marking scheme for MET 2026?
Ans. The marking scheme depends on the type of program:
For ME / M.Tech courses:
- Correct MCQ: +4 marks
- Incorrect MCQ: −1 mark
- Unattempted MCQ: 0 marks
- NAT Questions: +4 for correct, 0 for wrong or unattempted
For all other programs:
- No negative marking for any question.
- Marks are awarded only for correct answers.
- Final scores depend entirely on the number of correct responses.
Ques. Are NAT questions penalized for incorrect answers in MET 2026?
Ans. For ME/M.Tech courses, NAT questions carry +4 marks for correct answers and 0 marks for wrong/unattempted answers.
- For all other programs, NAT questions have no negative marking.
MET Exam Pattern 2026: Subject-Wise Syllabus
The syllabus for all the programs offered by the Manipal Academy of Higher Education is different. There is no complete, exhaustive guidance provided by MAHE for the syllabus of all kinds of subjects under all types of courses.
However, while going for MET 2026, you should keep the following two things in mind:
- For the UG course, syllabus of MET will cover the topics from the Class 12 level.
- For the PG course, syllabus of MET will cover the topics that are covered up to the respective graduation degree.
| Subject | Main Topics |
|---|---|
| Physics |
|
| Chemistry |
|
| Mathematics |
|
| English |
|
MET Exam Pattern 2026: Subject-Wise Weightage
The MET exam question paper includes four sections: Mathematics, Physics, Chemistry, and English.
- Mathematics carries the highest weightage with 20 questions & 80 marks, contributing about 33% of the total marks.
- Physics and Chemistry each have 15 questions & 60 marks, making up 25% each.
- English includes 10 questions & 40 marks, contributing the remaining 17%.
| Section / Subject | Type of Questions | No. of Questions | Marks per Question | Total Marks | Approx. Weightage (%) |
|---|---|---|---|---|---|
| Mathematics | 15 MCQs + 5 NATs | 20 | 4 | 80 | 33% |
| Physics | 10 MCQs + 5 NATs | 15 | 4 | 60 | 25% |
| Chemistry | 10 MCQs + 5 NATs | 15 | 4 | 60 | 25% |
| English | 10 MCQs | 10 | 4 | 40 | 17% |
| Total | — | 60 | — | 240 | 100% |
MET Exam Pattern 2026: Important Books
Some of the important books for students who are appearing for the MET Exam 2026 are given below in the table:
| Subject | Book Title | Author |
|---|---|---|
| Physics | Concepts of Physics | H.C. Verma |
| Understanding Physics | D.C. Pandey | |
| Electricity and Magnetism | D.C. Pandey | |
| Chemistry | Organic Chemistry | Morrison & Boyd |
| Physical Chemistry | P. Bahadur | |
| Inorganic Chemistry | J.D. Lee | |
| Mathematics | Problems in Calculus | S.L. Loney |
| Higher Algebra | Hall & Knight | |
| Trigonometry | I.A. Maron | |
| English | Wren & Martin's High School English Grammar & Composition | Wren & Martin |
MET Exam Preparation Tips
The table below shows the MET exam preparation tips for those who are preparing for the MET Exam.
| Section | Topics to Focus | Strategy / Tips | Suggested Hours per Week |
|---|---|---|---|
| Mathematics |
|
| 10–12 hours |
| Physics |
|
| 8–10 hours |
| Chemistry |
|
| 7–9 hours |
| English |
|
| 4–5 hours |
| General Strategy |
|
| 3–5 hours (mock tests) |
| Revision & Practice |
|
| 6–8 hours |
FAQs for MET Exam Pattern
Ques. What is the last date for Met registration 2026?
Ans. The registration date and last date for the MET exam for 2026 are given below in the table:
| Aspect | Details |
|---|---|
| Exam Name | Manipal Entrance Test (MET) 2026 |
| Application Form | Available online at manipal.edu |
| Registration Deadline (Phase 1) | March 15, 2026 |
| Exam Mode | Online (Computer-Based Test) |
| Exam Phases | Two phases: April 2026 and May 2026 |
Ques. What is the pattern of the MET exam?
Ans. The MET exam pattern 2026 is online and computer-based. The MET exam is conducted for 2 hours, and you are supposed to answer a total of 60 questions.
| Section | Type of Questions | Number of Questions | Total Marks |
|---|---|---|---|
| Mathematics | 15 MCQs + 5 NATs | 20 | 80 |
| Physics | 10 MCQs + 5 NATs | 15 | 60 |
| Chemistry | 10 MCQs + 5 NATs | 15 | 60 |
| English | 10 MCQs | 10 | 40 |
| Total | MCQs + NATs | 60 | 240 |
Ques. Is 70 marks good in MET?
Ans. In the MET 2026 exam, scoring 70 marks out of 240 may raise questions about competitiveness. The table below gives an overview of how different score ranges are generally assessed.
| Score in MET | Assessment | Remarks |
|---|---|---|
| 70 / 240 | Below Average | It may not be enough for top branches like Computer Science or Electronics. |
| 120–150 / 240 | Average | Can secure admission in some less competitive branches or campuses. |
| 180–200 / 240 | Good | Competitive for the most popular branches. |
| 200+ / 240 | Excellent | Likely to secure the top preferred branches and campuses. |
Ques. Is the MET exam conducted twice a year?
Ans. Yes, the MET exam will be conducted twice a year in 2026. The details are given below:
| Phase | Tentative Exam Month | Mode | Key Points |
|---|---|---|---|
| Phase 1 | April 2026 | Online (CBT) | First opportunity to take the exam; scores considered for admission. |
| Phase 2 | May 2026 | Online (CBT) | Second opportunityYou can improve scores or take the exam if you missed Phase 1. |
Ques. Is met tougher than JEE4?
Ans. The table below shows the difference between MET and JEE4:
| Aspect | MET Exam | JEE Main / JEE4 |
|---|---|---|
| Difficulty Level | Moderate | Moderate to Difficult |
| Focus Areas | Application-based questions, problem-solving | Conceptual understanding, deep problem-solving |
| Syllabus | Mathematics, Physics, Chemistry, English | Mathematics, Physics, Chemistry |
| Negative Marking | Yes (for MCQs only) | Yes (for MCQs) |
| Duration | 2 hours | 3 hours |
| Mode | Online (Computer-Based Test) | Online (Computer-Based Test) |
| Competition | Primarily for Manipal University admissions | National level: admissions in NITs, IIITs, etc. |
| Overall Toughness | Easier compared to JEE | Tougher, requires higher conceptual clarity |






Comments