NEET SS 2024 DrNB Medical Gastroenterology Paper 3 Question Paper with Solutions PDF is available for download. NEET SS 2024 exam is conducted by the National Board of Examinations in Medical Sciences (NBEMS). NEET SS 2024 question paper consists of 10 questions to be attempted in 3 hours. The paper is divided into broad-specialty topics (40%) and super-specialty topics (60%).
You can download NEET SS 2024 question paper with answer key and solutions PDF using the links given below.
NEET SS 2024 DrNB Medical Gastroenterology Paper 3 Question Paper with Solutions
| NEET SS 2024 DrNB Medical Gastroenterology Paper 3 Question Paper | Check Solutions |

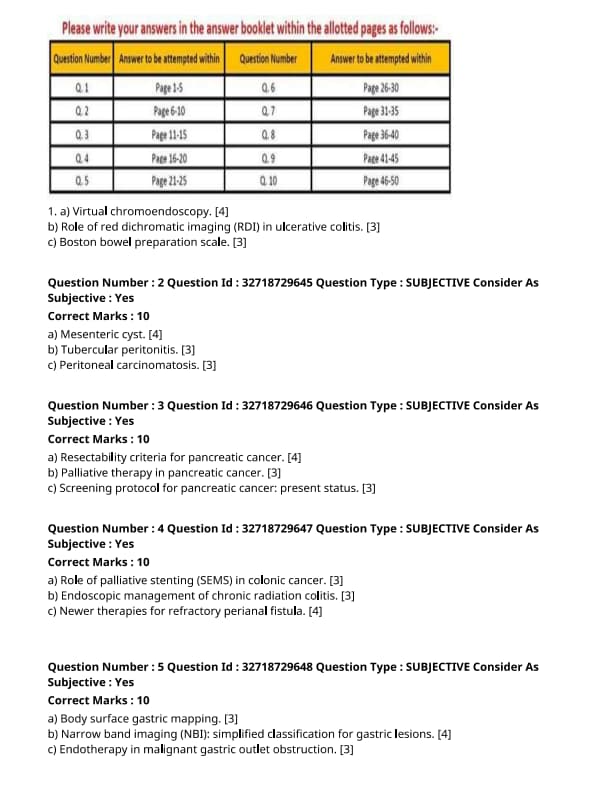
Virtual chromoendoscopy.
Role of red dichromatic imaging (RDI) in ulcerative colitis.
Boston bowel preparation scale.
Mesenteric cyst.
Tubercular peritonitis.
Peritoneal carcinomatosis.
Resectability criteria for pancreatic cancer.
Palliative therapy in pancreatic cancer.
Screening protocol for pancreatic cancer: present status.
Role of palliative stenting (SEMS) in colonic cancer.
Endoscopic management of chronic radiation colitis.
Newer therapies for refractory perianal fistula.
Body surface gastric mapping.
Narrow band imaging (NBI): simplified classification for gastric lesions.
Endotherapy in malignant gastric outlet obstruction.
HCV syndrome.
Tyrosine kinase inhibitors in GIST.
Role of EUS in the diagnosis of GIST.
Genomic profiling in GIST.
CA 19-9.
Diagnosing perihilar cholangiocarcinoma - Multimodality sampling.
Role of endobiliary ablation therapies in cholangiocarcinoma.
Describe histopathologics Findings of :
Celiac disease.
Describe histopathologics Findings of :
Tropical sprue.
Describe histopathologics Findings of :
Autoimmune enteropathy.
Describe histopathologics Findings of :
Abetalipoproteinemia.
Describe histopathologics Findings of :
Common variable immuno-deficiency (CVID).
Describe structures visualized in EUS at various GI stations:
i) GE junction.
ii) Pylorus.
ii) Pylorus.
EUS guided hepatico-gastrostomy.
Optoacoustic endoscopy of GI tract.
Management of IBD in pregnancy.
Immunoproliferative small intestinal disease (IPSID).









Comments