NEET SS 2024 DrNB Neuro Surgery Direct 6 years course Part 2 Paper 3 Question Paper with Solutions PDF is available for download. NEET SS 2024 exam is conducted by the National Board of Examinations in Medical Sciences (NBEMS). NEET SS 2024 question paper consists of 10 questions to be attempted in 3 hours. The paper is divided into broad-specialty topics (40%) and super-specialty topics (60%).
You can download NEET SS 2024 question paper with answer key and solutions PDF using the links given below.
NEET SS 2024 DrNB Neuro Surgery Direct 6 years course Part 2 Paper 3 Question Paper with Solutions
| NEET SS 2024 DrNB Neuro Surgery Direct 6 years course Part 2 Paper 3 Question Paper | Check Solutions |

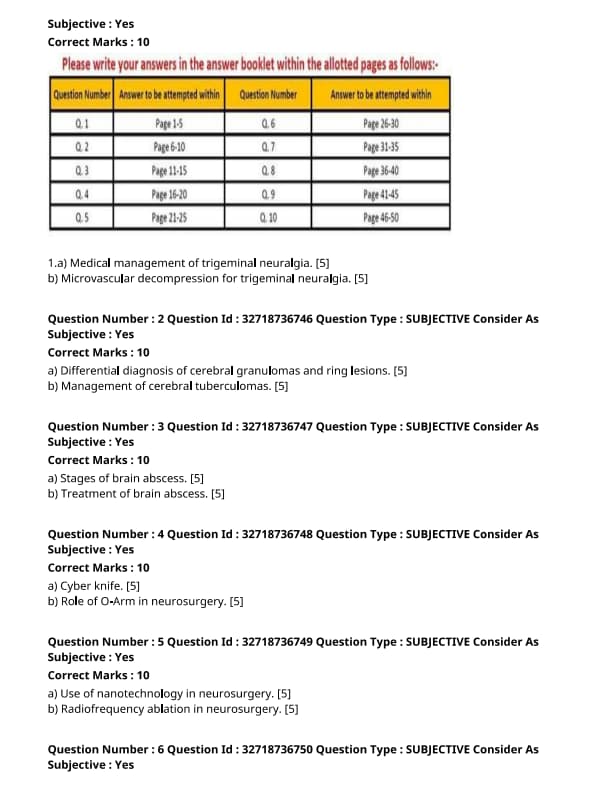
Medical management of trigeminal neuralgia.
View Solution
Trigeminal neuralgia is characterized by severe, stabbing pain along the trigeminal nerve. Medical management involves anticonvulsants and other pharmacological agents to control the pain.
Step 1: First-line Medications:
1. Carbamazepine: The most commonly used medication for trigeminal neuralgia, carbamazepine reduces nerve excitability by inhibiting sodium channels.
2. Oxcarbazepine: An alternative to carbamazepine, offering similar efficacy with fewer side effects.
Step 2: Second-line Medications:
1. Gabapentin and Pregabalin: These GABA analogs are effective in reducing pain when carbamazepine is not tolerated or effective.
2. Baclofen: A muscle relaxant that can help reduce pain by inhibiting the release of excitatory neurotransmitters in the central nervous system.
Step 3: Adjunctive Medications:
1. Tricyclic Antidepressants (TCAs): Amitriptyline may help reduce pain in some patients by altering neurotransmitter levels.
2. Topical Lidocaine: Topical anesthetics can provide temporary relief in some patients with localized pain. Quick Tip: For patients who do not respond to medical treatment, surgical options like microvascular decompression may be considered.
Microvascular decompression for trigeminal neuralgia.
View Solution
Microvascular decompression (MVD) is a surgical procedure performed in patients with trigeminal neuralgia who do not respond to medical therapy.
Step 1: Surgical Technique:
1. Craniotomy: A small opening is made in the skull to access the trigeminal nerve at the brainstem.
2. Vascular Decompression: The surgeon identifies and separates any blood vessels that are compressing the trigeminal nerve and places a small cushion (usually Teflon) to relieve the pressure on the nerve.
Step 2: Indications for MVD:
1. Failure of Medical Management: MVD is often performed when anticonvulsant medications are ineffective or cause unacceptable side effects.
2. Suitability for Surgery: Ideal candidates for MVD are those with clear vascular compression of the trigeminal nerve and no significant medical contraindications.
Step 3: Outcomes and Complications:
1. Success Rate: MVD is highly effective, with many patients experiencing long-term relief from pain.
2. Complications: Potential risks include facial weakness, hearing loss, cerebrospinal fluid leaks, and recurrence of neuralgia. Quick Tip: Microvascular decompression offers a long-term solution for trigeminal neuralgia and is particularly effective in patients with vascular compression of the trigeminal nerve.
Differential diagnosis of cerebral granulomas and ring lesions.
View Solution
Cerebral granulomas and ring lesions are often seen on imaging, particularly in the brain, and can be caused by a variety of conditions. Differentiating between these conditions is critical for appropriate management.
Step 1: Causes of Cerebral Granulomas and Ring Lesions:
1. Infectious Causes:
- Tuberculosis (TB): Cerebral tuberculomas, a form of granuloma, present as ring-enhancing lesions, particularly in immunocompromised individuals.
- Fungal Infections: Fungal diseases such as cryptococcosis or histoplasmosis can cause ring-enhancing lesions that are similar to granulomas.
- Parasitic Infections: Neurocysticercosis (pork tapeworm) is a common cause of ring-enhancing lesions.
- Bacterial Infections: Abscesses caused by bacterial infections, including pyogenic abscesses, can present with a similar ring-enhancing appearance.
2. Non-Infectious Causes:
- Neoplastic Lesions: Metastatic brain tumors, especially from the lung, breast, or colon, may present as ring-enhancing lesions.
- Autoimmune Diseases: Conditions such as sarcoidosis or granulomatosis with polyangiitis (Wegener's) can cause granulomas in the brain.
- Multiple Sclerosis (MS): MS may cause periventricular lesions that can enhance with contrast, appearing as ring lesions.
- Vascular Causes: In conditions like vasculitis, ring-enhancing lesions may develop as a result of inflammation of blood vessels.
Step 2: Imaging Findings:
1. CT/MRI: Ring-enhancing lesions are typically visible with contrast on CT or MRI. This enhancement is a result of inflammation and breakdown of the blood-brain barrier.
2. Biopsy or Culture: A biopsy may be needed to definitively diagnose the cause of the lesion, particularly if the etiology remains unclear after imaging studies. Quick Tip: Ring-enhancing lesions and granulomas can result from infections, autoimmune diseases, and neoplasms, so a detailed work-up is essential to identify the cause.
Management of cerebral tuberculomas.
View Solution
Cerebral tuberculomas are localized granulomatous lesions in the brain caused by tuberculosis. These lesions can present with neurological symptoms, and early diagnosis and treatment are crucial to prevent complications.
Step 1: Medical Management:
1. Antituberculous Therapy (ATT):
- The first-line treatment for cerebral tuberculomas is a combination of antituberculous drugs, including isoniazid, rifampicin, pyrazinamide, and ethambutol. The treatment duration is typically 6-12 months.
- Directly observed therapy (DOT) is preferred to ensure adherence and reduce the risk of drug resistance.
- Adjustments may be made if the patient shows resistance to certain drugs.
2. Corticosteroids:
- Steroids such as prednisone are often used to reduce inflammation and mass effect associated with the tuberculomas. This is particularly important in cases where the lesion causes significant symptoms like seizures or raised intracranial pressure.
Step 2: Surgical Management:
1. Surgical Resection:
- Surgery is considered if the tuberculomas are large, causing significant mass effect, seizures, or are not responsive to medical treatment.
- In cases where there is suspicion of abscess formation or if the lesion is in a location that makes it accessible, surgical intervention may be necessary.
Step 3: Monitoring and Follow-Up:
1. MRI/CT Scans:
- Periodic imaging is used to monitor the response to treatment and check for any recurrence of tuberculomas.
- In some cases, if the lesion shows signs of regression, a reduced frequency of imaging may be sufficient.
2. Long-Term Follow-Up:
- Follow-up with neurological assessments is essential to monitor for any new neurological deficits or recurrence of symptoms. Quick Tip: Prompt initiation of antituberculous therapy and corticosteroids is crucial in managing cerebral tuberculomas and preventing neurological complications.
Stages of brain abscess.
View Solution
Brain abscesses are localized infections within the brain tissue, often resulting from bacterial, fungal, or parasitic infections. The abscess develops in stages, with distinct phases of progression.
Step 1: Stages of Brain Abscess Formation:
1. Early Stage (Inflammatory Stage): This stage typically occurs within the first few days to weeks after infection. In this phase, a localized inflammatory response occurs at the site of infection, leading to edema (swelling) and the formation of a small collection of pus. The surrounding tissue may become necrotic.
2. Suppurative Stage (Pus Formation): As the infection progresses, the body responds by increasing the production of pus, which accumulates at the center of the abscess. This stage involves the formation of a thick, encapsulated collection of pus surrounded by inflammatory cells.
3. Mature Stage (Encapsulation): Over time, the abscess becomes encapsulated by a fibrous membrane. This barrier helps to limit the spread of infection, but the abscess continues to exert pressure on surrounding brain tissue. The center of the abscess remains filled with pus, which may be composed of dead tissue, bacteria, and inflammatory cells.
4. Resolution or Complication Stage: If untreated, the abscess may continue to grow, leading to increased intracranial pressure, and may cause permanent brain damage. In some cases, the abscess may resolve on its own or with treatment, but complications such as brain herniation or rupture into the ventricular system can occur. Quick Tip: Brain abscesses progress through distinct stages, and timely diagnosis and treatment are crucial to preventing complications.
Treatment of brain abscess.
View Solution
The treatment of a brain abscess involves a combination of medical and surgical interventions. The aim is to eradicate the infection, reduce intracranial pressure, and prevent further neurological damage.
Step 1: Medical Treatment:
1. Antibiotics: The first step in treating a brain abscess is the use of intravenous antibiotics to target the causative organisms. Broad-spectrum antibiotics are typically started initially, followed by more targeted therapy once the specific microorganism is identified. The treatment course may last for several weeks.
2. Antifungal or Antiparasitic Therapy: If the abscess is caused by fungi or parasites, specific antifungal or antiparasitic medications are prescribed. For example, in cases of fungal abscesses, drugs like amphotericin B may be used.
3. Steroids: Corticosteroids such as dexamethasone may be administered to reduce inflammation and edema surrounding the abscess, thus decreasing intracranial pressure.
4. Seizure Prophylaxis: Anticonvulsants may be used to prevent seizures, which are a common complication of brain abscesses.
Step 2: Surgical Treatment:
1. Abscess Drainage: If the abscess is large, or if the patient is not responding to medical treatment, surgical drainage may be required. This involves either aspiration of the pus using a needle or, in some cases, surgical resection of the abscess.
2. Craniotomy: In severe cases, a craniotomy may be performed to remove the abscess and reduce the pressure on the brain.
3. Monitoring: Post-surgical monitoring of intracranial pressure, neurological status, and infection markers is essential to prevent complications and ensure proper recovery.
Step 3: Post-treatment Care:
1. Rehabilitation: After the abscess is treated, patients may require rehabilitation to recover lost neurological function. This can include physical, occupational, and speech therapy.
2. Long-term Follow-up: Ongoing follow-up is necessary to ensure there is no recurrence of the infection and to manage any long-term neurological deficits. Quick Tip: Early diagnosis and a combination of antibiotic therapy and surgical drainage are key to effective treatment of brain abscesses.
Cyber knife.
View Solution
CyberKnife is a non-invasive, robotic radiosurgery system used to treat tumors and other medical conditions with high precision.
Step 1: Mechanism of Action:
CyberKnife uses a robotic arm to deliver precise radiation to the tumor or abnormal tissue from multiple angles. It tracks the tumor’s movement in real-time using X-ray imaging, allowing for accurate targeting while minimizing damage to surrounding healthy tissues.
Step 2: Indications:
1. Brain Tumors: CyberKnife is often used for treating brain tumors, including gliomas, meningiomas, and metastatic tumors.
2. Spinal Tumors: It is effective for treating both primary and metastatic tumors in the spine.
3. Other Applications: CyberKnife can also treat conditions such as arteriovenous malformations (AVMs), trigeminal neuralgia, and certain functional disorders.
Step 3: Advantages:
1. Non-invasive: CyberKnife eliminates the need for traditional surgery, reducing the risks of infection and recovery time.
2. Precision: It delivers high-dose radiation with millimeter precision, reducing exposure to healthy tissue. Quick Tip: CyberKnife is particularly beneficial for patients with inoperable tumors or those who are not candidates for traditional surgery.
Role of O-Arm in neurosurgery.
View Solution
The O-Arm is a mobile, intraoperative imaging system used in neurosurgery to provide real-time, 3D imaging during procedures.
Step 1: Functionality:
1. 3D Imaging: The O-Arm system provides high-resolution 3D imaging of the surgical area, allowing surgeons to visualize anatomy in real-time during the procedure.
2. Intraoperative Navigation: It helps guide the surgeon with precise navigation, reducing the risk of complications and improving accuracy during delicate procedures.
Step 2: Applications in Neurosurgery:
1. Spinal Surgery: The O-Arm is frequently used in spinal surgeries for accurate placement of screws, rods, and other implants.
2. Brain Surgery: It is also used in brain surgery to provide precise imaging of the brain, facilitating the removal of tumors or other lesions with minimal risk to surrounding tissues.
Step 3: Benefits:
1. Real-time Feedback: The O-Arm allows surgeons to assess their progress during surgery, reducing the likelihood of errors and improving surgical outcomes.
2. Minimized Incisions: By providing detailed imaging, the O-Arm enables surgeons to perform surgeries with smaller incisions, leading to quicker recovery times. Quick Tip: The O-Arm enhances the safety and precision of neurosurgical procedures, improving patient outcomes with real-time imaging and navigation.
Use of nanotechnology in neurosurgery.
View Solution
Nanotechnology in neurosurgery involves the application of nanoscale materials and devices to improve diagnosis, treatment, and monitoring of neurological disorders. It enables targeted therapy, precise surgery, and advanced imaging techniques.
Step 1: Applications of Nanotechnology in Neurosurgery:
1. Targeted Drug Delivery: Nanoparticles can be engineered to carry drugs directly to the brain, improving drug bioavailability while reducing side effects. This is especially useful in treating brain tumors, Alzheimer’s disease, and Parkinson’s disease.
2. Nanorobots for Surgery: Nanoscale robots can potentially be used for minimally invasive surgeries by performing precise procedures at the cellular level, reducing the risk of complications.
3. Neuroimaging: Nanoparticles such as quantum dots and magnetic nanoparticles are being used to enhance imaging modalities like MRI and PET, allowing for earlier and more accurate diagnosis of neurological conditions.
Step 2: Advantages of Nanotechnology:
1. Precision: Nanotechnology allows for more accurate targeting of treatment to specific brain areas, improving therapeutic outcomes.
2. Reduced Side Effects: By targeting only the diseased cells or tissues, nanotechnology minimizes the damage to healthy surrounding tissues, thus reducing side effects.
3. Better Diagnostic Tools: Enhanced imaging capabilities lead to earlier detection and better monitoring of neurological conditions.
Step 3: Challenges and Limitations:
1. Safety Concerns: The long-term effects of nanoparticles in the human body are not yet fully understood, and there are concerns regarding toxicity and immune response.
2. Regulatory Issues: The use of nanomaterials in humans requires extensive regulation to ensure safety and efficacy, which is still under development. Quick Tip: Nanotechnology holds great promise in neurosurgery, offering more precise treatments and better outcomes, but safety and regulatory challenges remain.
Radiofrequency ablation in neurosurgery.
View Solution
Radiofrequency ablation (RFA) is a minimally invasive procedure used in neurosurgery to treat various neurological conditions by using heat generated from radiofrequency waves to target and destroy abnormal tissues.
Step 1: Mechanism of Action:
1. Heat Generation: RFA works by passing radiofrequency energy through an electrode, which generates heat that coagulates tissue, leading to the destruction of abnormal cells.
2. Targeted Ablation: The procedure is typically used for targeting specific brain areas, such as tumors, epileptic foci, or other abnormal tissues, with high precision.
Step 2: Clinical Applications:
1. Brain Tumors: RFA is used to treat small or inaccessible brain tumors, providing a less invasive alternative to traditional surgery.
2. Epilepsy: In patients with drug-resistant epilepsy, RFA is used to ablate epileptic foci, thereby reducing the frequency and severity of seizures.
3. Trigeminal Neuralgia: RFA is often used to treat trigeminal neuralgia by selectively ablating the nerve fibers responsible for the pain, offering relief in patients who do not respond to medications.
Step 3: Advantages of RFA:
1. Minimally Invasive: RFA can be performed through small incisions or even through endoscopic techniques, reducing patient recovery time and complications.
2. Reduced Risk: Compared to traditional surgery, RFA carries less risk of infection, bleeding, and other complications.
3. Effectiveness in Inoperable Cases: RFA provides a treatment option for patients with tumors or conditions in difficult-to-reach locations where conventional surgery is not feasible.
Step 4: Limitations and Challenges:
1. Incomplete Ablation: In some cases, RFA may not completely ablate the target tissue, leading to recurrence of symptoms or disease.
2. Tissue Necrosis: The heat from RFA can cause collateral tissue damage if not precisely controlled, which can lead to adverse effects. Quick Tip: Radiofrequency ablation is a valuable tool in neurosurgery for treating brain tumors and neurological disorders, offering a less invasive option with fewer complications.
Vagal nerve stimulation.
View Solution
Vagal nerve stimulation (VNS) is a treatment option for patients with epilepsy who do not respond to medical management.
Step 1: Mechanism of Action:
VNS involves the implantation of a small pulse generator under the skin in the chest, which sends electrical impulses to the vagus nerve. These impulses travel to the brain, potentially reducing the frequency of seizures.
Step 2: Indications for VNS:
1. Refractory Epilepsy: VNS is commonly used in patients with focal or generalized epilepsy who do not respond well to antiepileptic drugs.
2. Ineligible for Surgery: It is also an option for patients who are not candidates for surgery due to the location of the seizure focus or other contraindications.
Step 3: Procedure and Follow-Up:
1. Implantation: The procedure involves placing an electrode around the left vagus nerve, which is connected to a pulse generator implanted in the chest.
2. Device Programming: The device is programmed to send electrical pulses at regular intervals, and the settings are adjusted based on the patient's response. Quick Tip: VNS can significantly reduce seizure frequency, though it may not completely eliminate seizures. Regular follow-up is required to adjust device settings.
Types of epilepsy surgery.
View Solution
Epilepsy surgery is considered for patients who have drug-resistant epilepsy and where seizures cannot be controlled with medications. Several types of surgical options exist, depending on the type of epilepsy and the location of the seizures.
Step 1: Resective Surgery:
1. Focal Cortical Resection: This involves removing the part of the brain that is responsible for generating seizures. It is typically performed for patients with a single, localized focus of seizures.
2. Hemispherectomy: In cases of severe, generalized epilepsy that affects one hemisphere of the brain, part or all of one hemisphere may be removed to stop seizures.
Step 2: Disconnective Surgery:
1. Corpus Callosotomy: This involves cutting the corpus callosum, which connects the two halves of the brain, to prevent seizure spread between hemispheres.
2. Multiple Subpial Transection: Small cuts are made in the brain to interrupt the spread of seizure activity without removing any tissue.
Step 3: Laser Ablation:
1. Laser Interstitial Thermal Therapy (LITT): LITT is a minimally invasive procedure that uses a laser to ablate the seizure focus. It is typically used when resective surgery is not possible. Quick Tip: Epilepsy surgery can provide significant relief for patients with refractory epilepsy and is considered after a thorough assessment and failure of medical management.
Magnetic resonance spectroscopy.
View Solution
Magnetic Resonance Spectroscopy (MRS) is an advanced imaging technique that provides biochemical information about tissues, particularly the brain, by measuring the levels of metabolites.
Step 1: Principles of MRS:
1. Metabolite Measurement: MRS detects the concentration of metabolites such as N-acetylaspartate (NAA), choline, creatine, and lactate. These metabolites reflect brain activity and cellular processes.
2. Non-Invasive Imaging: It is a non-invasive technique that uses magnetic fields and radio waves to gather data about the chemical composition of tissues, often used alongside MRI.
Step 2: Clinical Applications:
1. Brain Tumors: MRS helps differentiate malignant from benign tumors based on the metabolic signature.
2. Epilepsy: It is used to locate epileptic foci by identifying abnormal metabolic activity in specific regions of the brain.
3. Neurodegenerative Diseases: MRS can detect metabolic changes in conditions such as Alzheimer's disease, multiple sclerosis, and Parkinson's disease.
4. Stroke: MRS is used in assessing ischemic changes and monitoring recovery after a stroke.
Step 3: Limitations of MRS:
1. Spatial Resolution: MRS typically provides lower spatial resolution compared to conventional MRI, limiting its ability to accurately assess small or subtle lesions.
2. Technical Expertise: MRS requires advanced equipment and expertise to perform and interpret the results accurately. Quick Tip: MRS provides critical metabolic information that can aid in diagnosing and managing neurological disorders, particularly brain tumors and epilepsy.
Magnetic resonance tractography.
View Solution
Magnetic Resonance Tractography (MRT) is an advanced MRI technique that visualizes the white matter tracts in the brain, helping to assess the integrity and orientation of neural pathways.
Step 1: Principles of MRT:
1. Diffusion Tensor Imaging (DTI): MRT is based on DTI, which measures the diffusion of water molecules along the white matter fibers. It tracks the direction of the fibers, allowing for visualization of neural connections.
2. Fiber Tracking: The technique creates 3D maps of white matter pathways, providing detailed insight into brain networks and connectivity.
Step 2: Clinical Applications:
1. Pre-Surgical Planning: MRT is commonly used in the planning of neurosurgery to avoid important fiber tracts, minimizing the risk of functional deficits post-surgery.
2. Stroke Rehabilitation: It is used to assess the extent of white matter damage after a stroke and to monitor the recovery of neural connections.
3. Multiple Sclerosis: MRT helps track the progression of white matter damage in MS, offering insights into disease activity and treatment effects.
4. Neurodegenerative Diseases: In diseases like Alzheimer's, where there is progressive degeneration of white matter, MRT can assist in monitoring disease progression.
Step 3: Limitations of MRT:
1. Resolution: MRT has limited spatial resolution compared to other imaging modalities, making it difficult to visualize small or subtle abnormalities.
2. Technical Complexity: The technique requires specialized software and expertise for accurate interpretation, and the quality of images can be affected by movement artifacts. Quick Tip: Magnetic resonance tractography is a valuable tool for visualizing and evaluating white matter pathways, essential for surgical planning and monitoring brain diseases.
Flow diverters.
View Solution
Flow diverters are a type of endovascular device used to treat complex intracranial aneurysms by redirecting blood flow away from the aneurysm, allowing the vessel to remodel and reducing the pressure inside the aneurysm.
Step 1: Mechanism of Action:
1. Stent-like Structure: Flow diverters are essentially stent-like devices that are placed inside the parent artery of the aneurysm. The device covers the neck of the aneurysm, blocking blood flow into it.
2. Redirection of Flow: By diverting blood flow away from the aneurysm sac, flow diverters allow the blood vessel to remodel itself over time, reducing pressure inside the aneurysm and encouraging thrombosis (clot formation).
3. Pressure Reduction: The diversion of blood flow over time results in the aneurysm gradually closing off as the clot fills the sac, reducing the risk of rupture.
Step 2: Indications for Use:
1. Complex Aneurysms: Flow diverters are especially effective in treating large, wide-necked, or fusiform aneurysms that are difficult to treat with traditional techniques such as coiling or clipping.
2. Unruptured Aneurysms: Flow diverters are typically used for unruptured aneurysms that have a high risk of rupture and are located in challenging areas.
3. Recurrent Aneurysms: In cases where an aneurysm has recurred after previous treatments, flow diverters can provide an alternative solution.
Step 3: Benefits and Limitations:
1. Benefits: Flow diverters are a minimally invasive option that offers high rates of aneurysm obliteration. They are effective for treating aneurysms in difficult-to-reach locations like the internal carotid artery.
2. Limitations: The procedure carries some risks, including thromboembolic complications (e.g., stroke), delayed rupture, and the need for long-term antiplatelet therapy. Careful patient selection is crucial. Quick Tip: Flow diverters are a highly effective treatment for complex aneurysms but require careful monitoring to minimize complications such as thrombosis.
Indications for carotid endarterectomy.
View Solution
Carotid endarterectomy (CEA) is a surgical procedure used to treat carotid artery disease and prevent stroke. It involves removing plaque buildup from the carotid arteries to restore normal blood flow to the brain.
Step 1: Indications for Carotid Endarterectomy:
1. Symptomatic Carotid Stenosis: CEA is strongly indicated in patients with symptomatic carotid stenosis (i.e., those who have had a transient ischemic attack (TIA) or stroke) with a stenosis of 50% or greater. It reduces the risk of recurrent stroke.
2. Asymptomatic Carotid Stenosis: CEA can also be performed in patients with asymptomatic carotid stenosis of 70% or more, depending on individual risk factors such as age, health status, and the presence of other cardiovascular conditions.
3. Contralateral Stroke: CEA is indicated in patients who have had a stroke on the opposite side of the carotid artery with severe stenosis (greater than 80%).
4. High Surgical Risk: In some cases, if the patient is at high surgical risk, carotid artery stenting (CAS) might be considered as an alternative.
Step 2: Contraindications to Carotid Endarterectomy:
1. Severe Medical Comorbidities: Patients with severe systemic conditions such as end-stage renal disease or advanced cancer may not be candidates for CEA.
2. Total Carotid Artery Occlusion: CEA is not indicated in patients with total occlusion of the carotid artery, as the procedure will not improve cerebral blood flow.
3. High Surgical Risk: Patients who are not fit for surgery due to factors such as severe pulmonary disease or other severe comorbidities may not benefit from CEA.
Step 3: Preoperative Evaluation:
1. Imaging: Carotid ultrasound, CT angiography, or MR angiography is used to determine the degree of stenosis and identify the plaque characteristics.
2. Cardiac Risk Assessment: A thorough cardiac evaluation is necessary, as any underlying cardiovascular issues could affect surgical risk and recovery. Quick Tip: Carotid endarterectomy is a highly effective procedure for preventing stroke, but it is important to assess each patient carefully to determine the best treatment option.
Abciximab.
View Solution
Abciximab is a monoclonal antibody that is used as an antiplatelet drug, primarily to prevent thrombotic events during coronary angioplasty. It works by inhibiting the glycoprotein IIb/IIIa receptor on platelets, preventing fibrinogen binding and platelet aggregation.
Step 1: Mechanism of Action:
Abciximab binds to the glycoprotein IIb/IIIa receptor on platelets, which is necessary for platelet aggregation. By preventing this aggregation, abciximab reduces the formation of clots that can lead to heart attacks or strokes.
Step 2: Clinical Uses:
1. Coronary Artery Disease: Abciximab is used during percutaneous coronary interventions (PCI) to prevent thrombosis in high-risk patients.
2. Acute Coronary Syndromes: It is used in cases of unstable angina or non-ST-segment elevation myocardial infarction (NSTEMI) to reduce thrombotic complications.
Step 3: Side Effects:
1. Bleeding: The most common side effect of abciximab is bleeding, which can range from minor to life-threatening.
2. Thrombocytopenia: Rarely, abciximab can cause a drop in platelet count.
3. Hypotension: It can also cause low blood pressure, especially in patients who are already at risk.
Step 4: Contraindications:
1. Active Bleeding: Abciximab should not be used in patients who are actively bleeding.
2. Severe Hypertension: It is contraindicated in patients with uncontrolled hypertension due to the increased risk of bleeding. Quick Tip: Abciximab is an effective antiplatelet agent used during coronary procedures, but it must be used with caution due to the risk of bleeding and thrombocytopenia.
Newer anti-epileptic drugs.
View Solution
Newer anti-epileptic drugs (AEDs) have been developed to provide more effective treatment for epilepsy with fewer side effects and better pharmacokinetic profiles compared to older AEDs. These medications are used to control various types of seizures, especially in patients who do not respond to traditional treatments.
Step 1: Common Newer Anti-Epileptic Drugs:
1. Levetiracetam: Levetiracetam is a first-line treatment for epilepsy, effective for partial and generalized seizures. It works by inhibiting the release of excitatory neurotransmitters.
2. Lamotrigine: Lamotrigine is used for generalized and focal seizures and is known for its low side-effect profile. It works by stabilizing neuronal membranes through the inhibition of sodium channels.
3. Topiramate: Topiramate is effective for focal and generalized seizures and has multiple mechanisms of action, including the modulation of glutamate receptors and GABA activity.
4. Gabapentin: Gabapentin is often used as an adjunct therapy for partial seizures and neuropathic pain. It affects calcium channels, which helps in reducing excitability in neurons.
5. Zonisamide: Zonisamide is another broad-spectrum AED that blocks sodium and calcium channels, making it effective for partial and generalized seizures.
Step 2: Advantages of Newer AEDs:
1. Fewer Side Effects: Newer AEDs tend to have fewer side effects compared to older medications like phenytoin or carbamazepine.
2. Minimal Drug Interactions: Many newer AEDs have fewer interactions with other medications, making them ideal for patients on multiple drugs.
3. Better Tolerability: Newer AEDs are often better tolerated, with less sedation, cognitive impairment, or dizziness compared to older drugs.
Step 3: Limitations:
1. Cost: Newer AEDs can be more expensive than traditional medications, which may limit their accessibility.
2. Long-Term Safety: While these drugs are effective, their long-term safety profile is still being evaluated.
3. Side Effects: Despite improvements, newer AEDs can still cause side effects like dizziness, fatigue, and cognitive difficulties in some patients. Quick Tip: Newer anti-epileptic drugs are an important advancement in the treatment of epilepsy, providing better seizure control with fewer side effects, though their long-term effects and costs should be considered.
Medical management of Pott’s spine (TB).
View Solution
Step 1: Understanding Pott’s Spine:
Pott’s spine refers to tuberculosis (TB) of the spine, a form of osteomyelitis that primarily affects the vertebral bodies. It can lead to serious complications such as spinal deformities, neurological deficits, and abscess formation.
Step 2: Medical Management of Pott’s Spine:
1. Anti-Tuberculosis Therapy: The cornerstone of treatment is a combination of first-line anti-TB drugs, including Rifampicin, Isoniazid, Pyrazinamide, and Ethambutol. The standard treatment duration is 6-12 months, depending on clinical response.
2. Rest and Rehabilitation: Adequate bed rest is advised to limit further damage to the spine. Physical therapy may be necessary for rehabilitation, especially when neurological deficits are present.
3. Pain Management: Analgesics, including NSAIDs and corticosteroids, can be used for pain control and to reduce inflammation.
4. Nutritional Support: Proper nutrition plays a key role in boosting the immune system and supporting recovery from TB.
5. Monitoring for Side Effects: Regular follow-up is essential to monitor for potential drug side effects and to ensure treatment efficacy. Additional imaging or tests may be required to evaluate the condition of the spine. Quick Tip: Anti-TB drugs are essential for treating Pott’s spine, and early detection and treatment are crucial to preventing long-term complications.
Surgical management of Pott’s spine (TB).
View Solution
Step 1: Indications for Surgery in Pott’s Spine:
Surgical intervention is indicated when there are complications or inadequate response to medical therapy. Common indications include:
1. Neurological Deficits: Progressive weakness, numbness, or paralysis caused by spinal cord compression.
2. Spinal Deformity: Severe kyphosis or scoliosis that affects posture or causes pain.
3. Abscess Formation: Large paraspinal or epidural abscesses causing compression of the spinal cord.
4. Failure of Medical Therapy: In cases where medical management fails to control the infection or symptoms.
Step 2: Surgical Procedures:
1. Debridement and Drainage: Surgical removal of necrotic tissue and drainage of abscesses are performed in cases of paraspinal or epidural collections.
2. Spinal Fusion: In cases of instability or severe deformity, spinal fusion may be performed to stabilize the affected vertebrae and prevent further collapse.
3. Decompression Surgery: If the spinal cord is compressed, decompression surgery is performed to relieve pressure and prevent further neurological damage.
4. Postoperative Care: After surgery, the patient is typically immobilized with a brace, and rehabilitation is initiated to help recover spinal function and mobility. Quick Tip: Surgery in Pott’s spine is usually reserved for patients with neurological impairment, significant deformity, or abscess formation, and it should be followed by close monitoring and rehabilitation.





Comments