NEET SS 2024 DrNB Neuro Anaesthesia Paper 3 Question Paper with Solutions PDF is available for download. NEET SS 2024 exam is conducted by the National Board of Examinations in Medical Sciences (NBEMS). NEET SS 2024 question paper consists of 10 questions to be attempted in 3 hours. The paper is divided into broad-specialty topics (40%) and super-specialty topics (60%).
You can download NEET SS 2024 question paper with answer key and solutions PDF using the links given below.
NEET SS 2024 DrNB Neuro Anaesthesia Paper 3 Question Paper with Solutions
| NEET SS 2024 DrNB Neuro Anaesthesia Paper 3 Question Paper | Check Solutions |

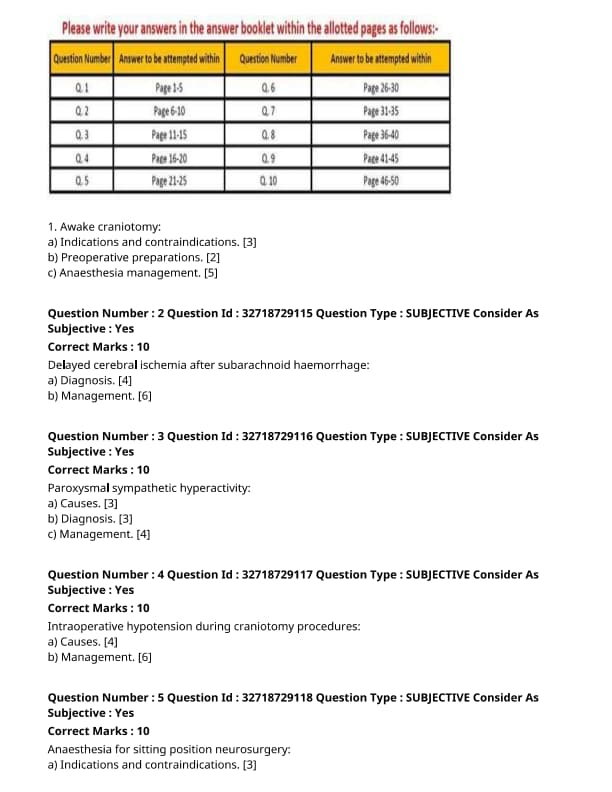
Indications and contraindications of awake craniotomy.
View Solution
Awake craniotomy is a procedure performed with the patient awake during parts of the surgery, allowing for real-time monitoring of brain functions.
Step 1: Indications:
1. Tumors Near Critical Brain Areas: Awake craniotomy is used for tumors located in areas that control language, motor functions, and other critical functions, to minimize damage to these areas.
2. Epilepsy Surgery: It is often used in patients with epilepsy, especially those with focal seizures, to map out seizure foci and avoid brain areas responsible for vital functions.
3. Brain Mapping: Awake craniotomy allows for direct cortical mapping and stimulation to help identify areas responsible for speech, motor functions, and sensory perception.
Step 2: Contraindications:
1. Uncooperative Patients: Awake craniotomy requires the patient to be awake and cooperative, so patients with cognitive impairments or who cannot follow instructions are not suitable candidates.
2. Severe Medical Conditions: Patients with significant comorbidities such as uncontrolled hypertension, bleeding disorders, or respiratory issues may not be candidates for awake craniotomy.
3. Tumors Inaccessible to Mapping: If the tumor is in a location that cannot be safely reached for mapping or resection, awake craniotomy may not be indicated. Quick Tip: Awake craniotomy is particularly valuable for surgeries in brain regions responsible for vital functions like speech and movement.
Preoperative preparations for awake craniotomy.
View Solution
Preoperative preparation for awake craniotomy involves both the physical and psychological preparation of the patient.
Step 1: Patient Evaluation:
1. Preoperative Imaging: Detailed imaging studies (MRI, CT) are performed to locate the tumor and assess its proximity to critical areas of the brain.
2. Neurological Assessment: A thorough neurological exam, including cognitive and motor function assessments, is necessary to identify baseline functions.
Step 2: Psychological Preparation:
1. Patient Education: The patient should be fully informed about the procedure, what to expect, and the importance of staying awake and responsive during the surgery.
2. Psychological Support: Many patients may feel anxious about being awake during the procedure, so providing psychological support and reassurance is crucial.
Step 3: Physical Preparation:
1. Anesthesia Consultation: A consultation with the anesthesia team is essential to determine the appropriate sedation level for the procedure.
2. Preoperative Fasting: The patient should fast for a prescribed period before surgery to minimize the risk of aspiration. Quick Tip: Proper preoperative preparation, including psychological support and patient education, is crucial to ensure patient cooperation during awake craniotomy.
Anaesthesia management in awake craniotomy.
View Solution
Anaesthesia management in awake craniotomy aims to provide adequate sedation and pain control while ensuring the patient remains alert and responsive for brain mapping.
Step 1: Sedation and Analgesia:
1. Conscious Sedation: A combination of local anesthesia and sedative medications (e.g., midazolam, dexmedetomidine) is used to keep the patient relaxed but responsive.
2. Pain Control: Local anesthesia is administered to the scalp and surgical area to provide pain relief during the procedure.
3. Light Anesthesia: The anesthesia team may maintain a light level of general anesthesia if needed, to prevent discomfort but still allow the patient to cooperate with the surgical team.
Step 2: Intraoperative Monitoring:
1. Neurological Monitoring: Continuous monitoring of the patient's neurological status is essential. The patient may be asked to perform tasks such as speaking or moving to assess brain function during surgery.
2. Vital Sign Monitoring: Blood pressure, heart rate, and oxygen levels are closely monitored to ensure the patient’s safety throughout the procedure.
Step 3: Communication and Adjustments:
1. Communication with the Patient: The surgical team must communicate with the patient regularly to ensure cooperation and to assess responses to stimulation.
2. Adjusting Sedation Levels: Sedation levels may be adjusted as needed to keep the patient awake but comfortable during critical parts of the surgery. Quick Tip: Effective anesthesia management requires a balance between adequate sedation for patient comfort and the ability to maintain alertness for neurological monitoring.
Delayed cerebral ischemia after subarachnoid hemorrhage:
Diagnosis
View Solution
Delayed cerebral ischemia (DCI) is a common and serious complication after subarachnoid hemorrhage (SAH). It occurs several days after the initial hemorrhage and can lead to significant morbidity or mortality if not properly diagnosed and managed.
Step 1: Clinical Features of DCI:
1. Timing of Onset: DCI typically develops between 3 and 14 days after the hemorrhage. This delayed onset distinguishes it from early complications like rebleeding.
2. Neurological Deterioration: Patients with DCI may show signs of progressive neurological deterioration, such as confusion, hemiparesis, or loss of consciousness.
3. Clinical Symptoms: Symptoms can include new-onset focal neurological deficits (e.g., weakness, aphasia), decreased level of consciousness, and seizures.
Step 2: Diagnostic Tools:
1. Neuroimaging:
- CT/MRI: Early CT or MRI scans may not detect ischemic changes, but subsequent imaging may reveal areas of hypodensity or infarction, especially in the watershed regions of the brain.
- CT Angiography (CTA): CTA can detect vasospasm, a common cause of DCI, by identifying areas of narrowed or occluded arteries.
- Magnetic Resonance Angiography (MRA): MRA can be used to assess the intracranial vasculature for vasospasm and identify areas at risk.
2. Cerebral Blood Flow Monitoring: Techniques such as transcranial Doppler (TCD) and brain tissue oxygen monitoring (PbtO2) are used to assess cerebral blood flow and oxygenation, helping to identify patients at risk for ischemia. Quick Tip: DCI is diagnosed through a combination of clinical monitoring, neuroimaging, and cerebral blood flow measurements, which helps identify at-risk patients early.
Delayed cerebral ischemia after subarachnoid hemorrhage:
Management
View Solution
Management of delayed cerebral ischemia (DCI) after subarachnoid hemorrhage (SAH) aims to prevent secondary brain injury, improve outcomes, and minimize neurological deficits.
Step 1: Medical Management:
1. Nimodipine:
- Nimodipine is a calcium channel blocker that has been shown to improve outcomes in patients with SAH. It is used to reduce the risk of vasospasm, which is a common cause of DCI.
- It is typically administered orally or via nasogastric tube in the early stages after SAH.
2. Blood Pressure Management:
- Maintaining adequate cerebral perfusion pressure (CPP) is essential in preventing DCI. This can be achieved by managing blood pressure, either by inducing mild hypertension (with drugs like norepinephrine) or by adjusting antihypertensive therapy as necessary.
- A target CPP is typically >60 mmHg.
3. Induced Hypertension:
- If vasospasm is detected, induced hypertension is often used to improve blood flow to ischemic regions of the brain. This involves using drugs to increase blood pressure temporarily, thus enhancing cerebral perfusion.
Step 2: Surgical Management:
1. Endovascular Therapy:
- In cases where severe vasospasm is identified and medical management fails, endovascular therapy, such as balloon angioplasty or intra-arterial vasodilator therapy, can be used to open up the narrowed vessels and improve cerebral blood flow.
2. Decompressive Surgery:
- In severe cases of DCI with significant mass effect, decompressive craniectomy may be performed to reduce intracranial pressure and prevent further brain injury.
Step 3: Monitoring and Supportive Care:
1. Neurological Monitoring:
- Continuous neurological monitoring is essential to detect early signs of deterioration. This includes the Glasgow Coma Scale (GCS) assessments and regular clinical evaluations for new neurological deficits.
2. Fluid Management:
- Careful fluid management is essential to maintain appropriate hydration and electrolyte balance, as both hypovolemia and hypervolemia can worsen cerebral ischemia.
Step 4: Preventing Complications:
1. Anticoagulation:
- Avoiding anticoagulation therapy in the acute phase is critical, as it can exacerbate bleeding and worsen outcomes. After stabilization, appropriate anticoagulation may be resumed based on individual risks.
2. Seizure Prophylaxis:
- Antiepileptic drugs are considered in cases of seizures or in patients with a higher risk of seizures due to DCI. Quick Tip: Managing DCI requires a multidisciplinary approach, including medical and surgical interventions, to optimize cerebral perfusion and prevent neurological deterioration.
Causes of Paroxysmal Sympathetic Hyperactivity.
View Solution
Paroxysmal sympathetic hyperactivity (PSH) is a syndrome characterized by episodes of excessive sympathetic nervous system activation. These episodes can result in hypertension, tachycardia, sweating, and agitation, and they typically occur in response to noxious stimuli.
Step 1: Causes of Paroxysmal Sympathetic Hyperactivity:
1. Neurological Injury: PSH can be triggered by traumatic brain injuries, particularly those affecting the brainstem or hypothalamus, which are involved in autonomic regulation.
2. Infections: Severe infections such as meningitis or encephalitis may also lead to PSH due to inflammation in the brain.
3. Stroke: Strokes, especially those affecting the brainstem or upper cervical spinal cord, are a common cause of PSH.
4. Toxins or Medications: Certain drugs, such as sympathomimetics (e.g., amphetamines), and toxins can overstimulate the sympathetic nervous system.
5. Severe Pain or Stress: Any form of severe stress, physical trauma, or pain can provoke a sympathetic response, especially in patients with predisposing conditions. Quick Tip: PSH is most commonly associated with neurological injuries, but it can also be triggered by infections, strokes, or external stimuli such as stress or pain.
Diagnosis of Paroxysmal Sympathetic Hyperactivity.
View Solution
The diagnosis of paroxysmal sympathetic hyperactivity (PSH) is primarily clinical, based on the recognition of characteristic signs and symptoms. Additional tests may be used to rule out other causes of sympathetic hyperactivity and to identify the underlying cause.
Step 1: Clinical Presentation:
1. Symptomatic Episodes: The patient exhibits sudden and recurrent episodes of sympathetic activation, such as increased heart rate (tachycardia), high blood pressure (hypertension), excessive sweating, fever, and restlessness. These episodes are typically transient and resolve once the triggering factor is addressed.
2. History and Physical Examination: A thorough medical history and physical examination are essential to identify potential triggers, such as recent neurological injury, infection, or trauma.
Step 2: Diagnostic Tests:
1. Blood Pressure Monitoring: Continuous or intermittent blood pressure monitoring may be used to assess the severity and frequency of hypertension episodes during paroxysms.
2. Heart Rate Monitoring: The patient may have episodes of tachycardia that correlate with the paroxysmal events.
3. Neuroimaging: MRI or CT scans of the brain may be performed to identify any neurological lesions, such as stroke or brain injury, that may be causing the sympathetic hyperactivity.
4. Blood Tests: Blood tests may be used to rule out infections or metabolic imbalances as contributing factors. Quick Tip: Diagnosis of PSH involves identifying characteristic episodes of sympathetic overactivity, with neuroimaging and blood tests used to identify underlying causes.
Management of Paroxysmal Sympathetic Hyperactivity.
View Solution
Management of paroxysmal sympathetic hyperactivity (PSH) focuses on controlling the acute sympathetic episodes, addressing the underlying cause, and preventing future episodes.
Step 1: Acute Management:
1. Sedation: Benzodiazepines, such as lorazepam or diazepam, are often used to sedate the patient and reduce the sympathetic overactivity during an episode.
2. Beta-blockers: Beta-blockers like propranolol can be used to control tachycardia and hypertension, common symptoms during the paroxysms.
3. Alpha-blockers: Alpha-adrenergic blockers, such as clonidine, may be used to reduce blood pressure and control sympathetic activation.
Step 2: Long-term Management:
1. Address Underlying Causes: The management of any underlying conditions, such as neurological injury, infection, or metabolic imbalances, is crucial for long-term control of PSH.
2. Sympathetic Nerve Block: In some cases, a sympathetic nerve block may be considered as a longer-term intervention to control the excessive sympathetic activity.
Step 3: Prevention of Future Episodes:
1. Pain Management: Effective control of pain, which can trigger PSH episodes, is essential in preventing recurrent episodes.
2. Management of Stress: Psychological support and stress management techniques, such as relaxation therapy or counseling, can help in reducing triggers of PSH. Quick Tip: Acute management of PSH involves controlling sympathetic activation with sedatives and beta-blockers, while addressing the underlying cause is key to long-term management.
Causes of intraoperative hypotension during craniotomy procedures.
View Solution
Intraoperative hypotension during craniotomy procedures is a serious concern that can lead to various complications, including poor tissue perfusion, brain ischemia, and delayed recovery. Several factors contribute to the development of hypotension during these procedures.
Step 1: Anesthesia-Related Causes:
1. Hypovolemia: The use of anesthetic agents, particularly general anesthetics, can cause vasodilation and a decrease in blood volume, leading to hypotension.
2. Vasodilation: Anesthetic drugs like isoflurane and sevoflurane can induce vasodilation, resulting in reduced systemic vascular resistance and blood pressure.
3. Epidural or Spinal Anesthesia: These anesthetic techniques can block sympathetic tone, causing a drop in blood pressure due to vasodilation and decreased cardiac output.
Step 2: Surgical Factors:
1. Positioning of the Patient: During craniotomy, the patient is often placed in positions that can affect venous return (e.g., head-up or reverse Trendelenburg), leading to reduced cardiac output and hypotension.
2. Blood Loss: Intraoperative blood loss, especially during skull and brain tissue dissection, can contribute to hypovolemia and subsequent hypotension.
3. CSF Drainage: Draining cerebrospinal fluid (CSF) during craniotomy can reduce the intracranial pressure, but it may also cause a decrease in cerebral perfusion pressure, leading to hypotension.
Step 3: Pre-existing Medical Conditions:
1. Cardiac Dysfunction: Patients with pre-existing cardiovascular conditions may have limited ability to compensate for the physiological stresses induced by anesthesia and surgery, leading to hypotension.
2. Endocrine Disorders: Conditions such as hypothyroidism or adrenal insufficiency can impair the body's response to stress, increasing the risk of hypotension during surgery. Quick Tip: Managing the risk of hypotension during craniotomy involves careful anesthetic management, monitoring of blood volume, and maintaining optimal positioning and hemodynamic support.
Management of intraoperative hypotension during craniotomy procedures.
View Solution
The management of intraoperative hypotension during craniotomy procedures involves prompt recognition and appropriate interventions to maintain adequate cerebral perfusion and prevent further complications. Treatment strategies include both pharmacological and non-pharmacological approaches.
Step 1: Monitoring and Assessment:
1. Continuous Blood Pressure Monitoring: Intraoperative blood pressure should be continuously monitored using an arterial line, especially in high-risk patients, to detect hypotension early.
2. Cerebral Perfusion Monitoring: Monitoring devices such as near-infrared spectroscopy (NIRS) can be used to assess cerebral oxygenation, helping guide treatment decisions.
3. Hemodynamic Parameters: It is essential to monitor cardiac output, heart rate, and central venous pressure to assess the cause of hypotension.
Step 2: Non-Pharmacological Management:
1. Positioning: The patient’s position should be optimized to enhance venous return, particularly during craniotomy. Positioning the head appropriately, avoiding excessive tilting, and ensuring proper neck alignment can help improve circulation.
2. Fluid Resuscitation: Intravenous fluids (e.g., crystalloids, colloids) should be administered to correct any volume deficits and restore blood pressure. A target mean arterial pressure (MAP) should be set based on the patient's condition and surgical requirements.
Step 3: Pharmacological Management:
1. Vasopressors: Drugs like phenylephrine, norepinephrine, or ephedrine can be used to increase systemic vascular resistance and raise blood pressure in the event of hypotension.
2. Inotropes: In cases where hypotension is due to reduced cardiac output, inotropes such as dobutamine may be used to enhance myocardial contractility.
3. Adjusting Anesthetic Agents: If hypotension is related to excessive vasodilation from anesthetics, adjustments should be made to the depth of anesthesia, or vasoconstrictors can be added to counteract the effects.
Step 4: Correction of Blood Loss:
1. Transfusion: If significant blood loss occurs during surgery, blood products (such as red blood cells, plasma, or platelets) should be administered to replace lost volume and improve oxygen-carrying capacity.
Step 5: Postoperative Care:
1. Continued Hemodynamic Monitoring: After the surgery, the patient should be monitored in the recovery room or intensive care unit for any signs of residual hypotension or complications.
2. Pain Control: Adequate pain control should be maintained to prevent hypotension associated with pain-induced sympathetic activation. Quick Tip: Effective management of intraoperative hypotension involves a combination of monitoring, fluid therapy, vasopressors, and appropriate adjustments to anesthetic agents to ensure adequate perfusion and prevent complications.
Indications and contraindications for anaesthesia in sitting position neurosurgery.
View Solution
Step 1: Indications for Sitting Position in Neurosurgery:
The sitting position is commonly used for certain types of neurosurgeries, especially those involving the posterior fossa or upper cervical spine. Indications include:
1. Posterior Fossa Surgeries: Such as tumor excision, decompression, and vascular surgeries where the surgeon needs direct access to the cerebellum or brainstem.
2. Cervical Spine Surgeries: Procedures involving the upper cervical spine, where visualization and access are enhanced by the sitting position.
3. Vascular Neurosurgery: Especially in cases like microvascular decompression, where the sitting position helps with venous drainage and reduces intracranial pressure.
Step 2: Contraindications for Sitting Position in Neurosurgery:
There are several contraindications where the sitting position should be avoided:
1. Patients with Increased Intracranial Pressure (ICP): The sitting position may exacerbate ICP, leading to complications such as brain herniation.
2. Cardiovascular Instability: Patients with severe hypotension or heart failure may not tolerate the sitting position.
3. Spinal Pathology: Certain spinal conditions may contraindicate the sitting position due to the risk of worsening spinal deformities or instability.
4. Obesity: Obese patients may not be able to safely assume the sitting position due to difficulties in positioning or breathing.
5. Airway Difficulties: The sitting position may pose challenges for intubation in patients with difficult airways. Quick Tip: Careful patient selection is essential when considering the sitting position for neurosurgery, as it can significantly impact surgical access and patient safety.
Advantages of anaesthesia in sitting position neurosurgery.
View Solution
Step 1: Advantages of Sitting Position in Neurosurgery:
1. Improved Surgical Exposure: The sitting position provides better access to the posterior fossa, cerebellum, and upper cervical spine, enhancing visibility for the surgeon.
2. Reduced Intracranial Pressure (ICP): The sitting position helps promote venous drainage, reducing intracranial pressure, which can be beneficial in surgeries involving the brainstem or posterior fossa.
3. Optimal Drainage of Blood: It improves blood drainage from the brain, reducing the risk of postoperative swelling or bleeding.
4. Ease of Handling Cerebrospinal Fluid (CSF): During surgeries involving CSF, the sitting position can make it easier to manage CSF leaks or access the spinal cord. Quick Tip: The sitting position can provide optimal surgical access and improved venous drainage, but it requires careful patient monitoring.
Complications of anaesthesia in sitting position neurosurgery.
View Solution
Step 1: Complications of Sitting Position in Neurosurgery:
1. Venous Air Embolism (VAE): One of the most serious complications, VAE can occur due to the sitting position, especially during craniotomies or spinal surgeries. It can lead to cardiovascular collapse if not managed promptly.
2. Postoperative Hypotension: The sitting position can cause a significant drop in blood pressure due to the pooling of blood in the lower extremities and reduced venous return.
3. Spinal Cord Injury: Improper positioning can lead to pressure on the spinal cord or nerve roots, causing neurological damage.
4. Complications from Inadequate Ventilation: The sitting position can restrict chest expansion, leading to breathing difficulties, especially in patients with pre-existing respiratory conditions. Quick Tip: Careful monitoring and positioning are crucial when using the sitting position in neurosurgery to minimize risks such as air embolism and hypotension.
Regression analysis.
View Solution
Regression analysis is a statistical method used for modeling the relationship between a dependent variable and one or more independent variables.
Step 1: Types of Regression Analysis:
1. Linear Regression: This method is used when the dependent variable is continuous, and the relationship with the independent variable(s) is linear.
2. Multiple Regression: This extends linear regression to include multiple independent variables.
3. Logistic Regression: Used when the dependent variable is categorical, commonly for binary outcomes (e.g., success/failure).
Step 2: Assumptions of Regression Analysis:
1. Linearity: There should be a linear relationship between the dependent and independent variables.
2. Independence: Observations should be independent of one another.
3. Homoscedasticity: The variance of errors should be constant across all levels of the independent variable. Quick Tip: Ensure the assumptions of regression analysis are met to avoid misleading results.
Forest plot.
View Solution
A forest plot is a graphical representation of the results of a meta-analysis or systematic review, showing individual study results and overall effects.
Step 1: Components of a Forest Plot:
1. Individual Study Results: Each study is represented by a horizontal line indicating the confidence interval (CI) of the estimate.
2. Point Estimate: The vertical line through each study’s result indicates the point estimate of the effect size.
3. Overall Effect: The combined effect of all studies is represented by a diamond shape, which shows the pooled estimate and its confidence interval.
Step 2: Interpretation of Forest Plot:
1. Significance: If the confidence intervals of a study or the overall estimate do not cross the vertical line of no effect (usually represented by zero or one, depending on the type of analysis), the result is statistically significant.
2. Heterogeneity: Variability in the effect estimates across studies is indicated by the width of the confidence intervals and the size of the individual study results. Quick Tip: Forest plots are useful for visualizing the overall effect size and consistency of results across studies.
Funnel plot.
View Solution
A funnel plot is used to assess publication bias in a meta-analysis by plotting study effect sizes against their standard errors.
Step 1: Structure of a Funnel Plot:
1. X-axis: The effect sizes (such as odds ratios, mean differences) of the individual studies are plotted on the x-axis.
2. Y-axis: The standard errors of the effect estimates are plotted on the y-axis.
3. Symmetry: A symmetrical funnel shape suggests no significant publication bias, whereas an asymmetrical shape indicates potential bias.
Step 2: Interpretation of Funnel Plot:
1. Symmetry and Bias: If studies with smaller sample sizes (larger standard errors) tend to show larger effects, it suggests publication bias.
2. Visual Inspection: A symmetrical funnel plot indicates no significant bias, while an asymmetrical funnel plot suggests selective reporting or publication bias. Quick Tip: Funnel plots are useful for detecting publication bias, but they should be interpreted with caution and in conjunction with other statistical tests.
A 12-month-old, 7.0 kg infant with Apert syndrome, bilateral coronal and lambdoidal craniosynostosis is scheduled for a total calvarial vault reconstruction.
Enumerate the perioperative concerns for this patient.
View Solution
A 12-month-old infant with Apert syndrome and craniosynostosis requires special perioperative considerations. Apert syndrome is a rare genetic condition characterized by craniosynostosis, syndactyly, and other developmental anomalies. The following perioperative concerns should be addressed:
Step 1: Airway Management:
1. Potential for Difficult Intubation: Due to craniosynostosis and abnormal head shape, airway management may be challenging. A difficult airway cart should be readily available.
2. Risk of Obstructive Apnea: These patients may have associated airway anomalies, including upper airway obstruction, which requires careful monitoring during anesthesia.
Step 2: Hemodynamic Considerations:
1. Blood Loss: Surgical correction of craniosynostosis may involve significant blood loss, particularly in infants. Preoperative assessment should include a baseline hemoglobin level and strategies for blood conservation.
2. Monitoring Blood Pressure: Maintaining stable blood pressure during surgery is crucial to avoid ischemic damage to the brain, especially considering the patient’s young age.
Step 3: Neurological Concerns:
1. Risk of Increased Intracranial Pressure (ICP): The cranial vault reconstruction surgery may elevate ICP. Close monitoring with a team experienced in managing high ICP is essential.
2. Seizure Risk: There may be an increased risk of seizures due to brain malformation, requiring appropriate prophylaxis and monitoring.
Step 4: Fluid and Electrolyte Management:
1. Dehydration Risk: Ensure adequate hydration during surgery, as infants are more susceptible to fluid imbalances.
2. Electrolyte Imbalances: Frequent monitoring of electrolytes should be performed to prevent disturbances during the procedure.
Step 5: Postoperative Care:
1. Pain Management: Postoperative pain should be effectively managed with age-appropriate analgesia, considering both narcotics and non-narcotic options.
2. Postoperative Monitoring: Due to the potential for complications like bleeding or respiratory distress, close monitoring in an intensive care unit (ICU) postoperatively is essential. Quick Tip: Effective management of airway, hemodynamics, and neurological status are key to ensuring a successful perioperative outcome for infants with Apert syndrome.
A 12-month-old, 7.0 kg infant with Apert syndrome, bilateral coronal and lambdoidal craniosynostosis is scheduled for a total calvarial vault reconstruction.
Discuss the blood loss management strategy for this patient.
View Solution
Blood loss is a significant concern during the total calvarial vault reconstruction in an infant with Apert syndrome and craniosynostosis. Careful planning and management are crucial to minimize complications.
Step 1: Preoperative Optimization:
1. Preoperative Hemoglobin Assessment: Check hemoglobin levels to assess the patient’s baseline status and determine if blood transfusion is required before surgery.
2. Blood Type and Crossmatch: Ensure availability of crossmatched blood or blood products, including packed red blood cells (PRBCs) and platelets, to address potential blood loss.
Step 2: Intraoperative Blood Loss Control:
1. Minimizing Blood Loss: Techniques such as controlled hypotension and hemostatic agents should be employed to minimize intraoperative bleeding.
2. Surgical Techniques: Employ meticulous surgical techniques to reduce unnecessary bleeding. Use of electrocautery or lasers may help in controlling bleeding during the procedure.
Step 3: Monitoring and Management:
1. Blood Loss Monitoring: Continuous monitoring of blood loss throughout the surgery is critical. Blood loss should be measured and recorded regularly.
2. Fluid and Electrolyte Management: Maintain the patient’s fluid balance to prevent hypovolemia. Crystalloid solutions, colloids, and blood products should be given as needed based on intraoperative blood loss.
Step 4: Postoperative Blood Loss Management:
1. Transfusion Protocol: Postoperative blood loss should be managed with appropriate transfusion of PRBCs and platelets to maintain hemodynamic stability and oxygen delivery.
2. Monitoring for Bleeding Complications: Postoperative drainage systems may be required to monitor ongoing bleeding, especially in the early postoperative period.
Step 5: Considerations in Infants:
1. Infant-Specific Concerns: In infants, the total blood volume is smaller, and blood loss can have a more profound effect. Careful attention to blood volume replacement and continuous monitoring is required. Quick Tip: Early identification of blood loss and timely interventions, including blood transfusion, are crucial in managing blood loss in infants undergoing cranial surgery.
Evoked potential monitoring:
Role in Neurosurgery.
View Solution
Evoked potential (EP) monitoring is widely used in neurosurgery to assess the function of neural pathways during surgery and detect potential damage to the nervous system in real-time. It helps ensure the safety of critical structures, minimizing the risk of postoperative neurological deficits.
Step 1: Role in Neurosurgery:
1. Monitoring Neural Integrity: EP monitoring allows for the continuous assessment of neural integrity during surgeries involving the brain, spine, and peripheral nerves. This is particularly crucial for procedures where critical motor and sensory pathways are at risk.
2. Detection of Ischemia or Injury: EPs can detect early signs of ischemia or neural injury, providing real-time feedback and allowing surgeons to adjust their technique to avoid or minimize damage.
3. Intraoperative Guidance: It helps in identifying the proximity of surgical instruments to critical neural structures, such as the spinal cord or brainstem, ensuring the safe removal of tumors or other lesions without compromising neurological function. Quick Tip: Evoked potential monitoring is an essential tool in neurosurgery to protect neural structures and ensure optimal surgical outcomes.
Evoked potential monitoring:
Types of Different Evoked Potential Monitoring Techniques.
View Solution
Evoked potential (EP) monitoring includes several techniques that assess the integrity of sensory and motor pathways. Each technique has its specific uses in various types of surgery, providing valuable real-time information to the surgical team.
Step 1: Types of Evoked Potential Monitoring Techniques:
1. Somatosensory Evoked Potentials (SSEPs): SSEPs measure the electrical activity of the brain in response to sensory stimuli (typically electrical stimulation of peripheral nerves). They are used to monitor the integrity of sensory pathways, especially during spinal surgeries or brainstem surgeries.
2. Motor Evoked Potentials (MEPs): MEPs are used to assess motor pathways by stimulating the motor cortex and measuring muscle responses. They are particularly useful for monitoring spinal cord function during surgeries such as scoliosis correction or spinal cord tumor resection.
3. Visual Evoked Potentials (VEPs): VEPs assess the visual pathway by stimulating the retina and recording responses from the visual cortex. These are used in surgeries involving the optic nerve or the visual cortex.
4. Auditory Evoked Potentials (AEPs): AEPs evaluate the auditory pathway by using sound stimuli and monitoring responses in the brainstem. They are used during brainstem or ear surgeries to monitor auditory nerve function. Quick Tip: Different types of evoked potentials (SSEPs, MEPs, VEPs, and AEPs) provide crucial information on the functional integrity of neural pathways during neurosurgery.
Evoked potential monitoring:
Anaesthesia Management for Evoked Potential Monitoring.
View Solution
Anaesthesia management during evoked potential (EP) monitoring plays a critical role in ensuring accurate readings. The anesthetic agents used must be chosen carefully to avoid interference with the neural responses being measured.
Step 1: Key Considerations in Anaesthesia:
1. Avoiding Neuromuscular Blockers: Neuromuscular blockers can interfere with the measurement of motor evoked potentials (MEPs) by preventing the muscle response to motor cortex stimulation. Therefore, their use should be avoided during surgeries that require MEP monitoring.
2. Light Anesthesia: Deep levels of anesthesia can suppress neural responses, making it difficult to interpret the EPs. A lighter level of anesthesia is preferred to maintain adequate neural responsiveness while ensuring patient comfort.
3. Use of Inhalational Agents: Inhalational anesthetic agents, such as sevoflurane, are commonly used during surgeries involving EP monitoring. These agents generally have less effect on somatosensory evoked potentials (SSEPs) compared to intravenous anesthetics.
4. Monitoring Depth of Anesthesia: Continuous monitoring of anesthetic depth is essential to ensure the patient remains at an appropriate level of anesthesia without compromising the quality of EP monitoring.
Step 2: Management of Surgical Stimuli:
1. Avoiding Excessive Pain or Stress: Surgical procedures that cause significant pain or stress may alter the evoked potentials. The anesthesia should be adjusted to minimize any stress response from the patient, which could interfere with EP signals.
2. Sedation for Comfort: Mild sedation may be required to keep the patient comfortable while maintaining sufficient neural activity for accurate EP readings.
Step 3: Recovery and Postoperative Care:
1. Careful Emergence from Anesthesia: The emergence from anesthesia should be managed carefully to prevent any sudden changes in neural function that could affect the EPs.
2. Postoperative Monitoring: Patients should be monitored postoperatively for any neurological deficits that may have been missed during the procedure. Quick Tip: Anesthesia management during evoked potential monitoring requires a careful balance to avoid interfering with neural responses while ensuring patient safety and comfort.
Criteria for diagnosis of brain death.
View Solution
Brain death is the irreversible cessation of all functions of the brain, including the brainstem. It is a legal and clinical definition of death, which is crucial for organ donation and end-of-life decisions. The criteria for diagnosing brain death involve several steps and clinical examinations to confirm the absence of brain activity.
Step 1: Clinical Criteria:
1. Clinical History: A clear history of an event leading to the loss of brain function, such as a severe head injury, stroke, or anoxia, must be established.
2. Neurological Examination: The patient must demonstrate an absence of all cerebral and brainstem activity. This includes:
- Coma or Unresponsiveness: No response to external stimuli, including pain, should be observed.
- Absent Cranial Nerve Reflexes: The absence of pupillary light reflex, corneal reflex, gag reflex, and response to facial stimuli is required.
- Absence of Respiratory Effort: The patient must be apneic, and no breathing should occur even when carbon dioxide levels rise. The apnea test is commonly used to confirm this.
Step 2: Confirmatory Tests:
1. Electroencephalogram (EEG): An absent EEG showing no electrical activity in the brain confirms brain death.
2. Cerebral Blood Flow Studies: Tests such as cerebral angiography or transcranial Doppler can confirm the absence of blood flow to the brain.
3. Brainstem Reflex Testing: Additional confirmatory tests such as an absent gag reflex, absent cough reflex, and no response to pain stimuli may be used to support the diagnosis.
Step 3: Legal and Ethical Considerations:
The diagnosis of brain death should be made according to legal and ethical guidelines, which may vary by country or state. A second examination by a different physician may be required after a set period of time to confirm the diagnosis. Quick Tip: The diagnosis of brain death is a critical clinical and ethical process, requiring thorough clinical examination and, if necessary, confirmatory tests to ensure the irreversible cessation of brain activity.
Postcardiac arrest neuroprognostication.
View Solution
Postcardiac arrest neuroprognostication is the process of determining the potential neurological outcomes of patients who survive a cardiac arrest. Accurate prognostication is important for guiding treatment decisions and end-of-life care.
Step 1: Initial Assessment:
1. Stabilization: After successful resuscitation, the first step is to stabilize the patient. This includes maintaining adequate oxygenation, blood pressure, and temperature control.
2. Neurological Examination: A thorough neurological examination is essential, including assessment of pupillary reflexes, corneal reflex, and motor responses to pain.
Step 2: Neurological Prognostic Indicators:
1. Glasgow Coma Scale (GCS): The GCS score is used to assess the level of consciousness. A GCS score of 3 (the lowest possible score) with no purposeful movement or response to verbal stimuli is a poor prognostic sign.
2. Corneal Reflex: The absence of corneal reflexes or pupillary light reflexes indicates severe brain injury and a poor prognosis.
3. Motor Response: The presence or absence of purposeful motor response (such as purposeful movement or withdrawal from pain) is a key indicator of neurological recovery.
4. Neurological Recovery Time: If the patient has not shown improvement within 24-72 hours of resuscitation, it suggests a poor neurological prognosis.
Step 3: Diagnostic Tests for Prognostication:
1. EEG (Electroencephalogram): The absence of cerebral electrical activity on EEG (isoelectric EEG) is strongly associated with poor neurological outcomes.
2. Somatosensory Evoked Potentials (SSEP): If there is no response in SSEP testing (especially in the cortical responses), it is a strong indicator of poor prognosis.
3. CT/MRI Brain Imaging: Imaging studies can reveal structural damage to the brain, including ischemic injury, which may help with prognosis determination.
Step 4: Predictive Models:
Several scoring systems, such as the "Hasselberg score," combine clinical, neurological, and laboratory findings to help predict outcomes following cardiac arrest. These models incorporate elements like age, the duration of cardiac arrest, initial rhythm, and post-arrest neurological findings.
Step 5: Ethical Considerations:
1. Family Involvement: Prognostication must be communicated clearly to the patient's family, and ethical considerations must guide decisions regarding life-sustaining treatments.
2. Shared Decision-Making: It is crucial to involve the family in discussions about prognosis and treatment options based on the neurological prognosis. Quick Tip: Postcardiac arrest neuroprognostication relies on clinical, electroencephalographic, and imaging data to assess the likelihood of neurological recovery, guiding decisions about treatment goals and end-of-life care.
Cerebral hyperperfusion syndrome.
View Solution
Step 1: Understanding Cerebral Hyperperfusion Syndrome:
Cerebral hyperperfusion syndrome (CHS) is a condition characterized by excessive blood flow to the brain, usually occurring after carotid endarterectomy or other cerebrovascular surgeries. It can lead to various neurological complications, including stroke or seizures.
Step 2: Pathophysiology:
CHS typically develops when blood flow to the brain increases excessively after surgery, overwhelming the autoregulatory mechanisms that normally adjust blood flow to the brain. This can cause:
1. Vascular Dilation: A sudden increase in blood flow leads to dilation of cerebral vessels, which may increase the risk of bleeding.
2. Edema: Hyperperfusion can lead to cerebral edema (swelling), which can cause increased intracranial pressure (ICP).
3. Ischemic Injury: Paradoxically, hyperperfusion can also lead to ischemia (lack of blood supply) in certain areas of the brain due to vascular damage or inadequate perfusion in other areas.
Step 3: Symptoms of Cerebral Hyperperfusion Syndrome:
1. Headache: Severe headache is a common presenting symptom, often occurring within hours or days after surgery.
2. Neurological Deficits: Patients may experience focal neurological deficits such as weakness, sensory loss, or visual disturbances.
3. Seizures: Seizures can be a manifestation of cerebral edema or ischemia resulting from hyperperfusion.
Step 4: Management:
1. Blood Pressure Control: The primary treatment for CHS involves controlling blood pressure to prevent further increases in cerebral blood flow.
2. Use of Vasodilators: Medications that dilate blood vessels can be used to reduce the pressure and blood flow to the brain.
3. Monitoring and Supportive Care: Close monitoring of neurological status and blood pressure is critical in managing CHS. Quick Tip: Cerebral hyperperfusion syndrome requires immediate intervention to prevent neurological damage and manage blood pressure to avoid further complications.
Circulatory arrest for flow control during cerebrovascular surgeries.
View Solution
Step 1: Understanding Circulatory Arrest in Neurosurgery:
Circulatory arrest involves stopping blood flow temporarily during cerebrovascular surgeries to facilitate procedures such as aneurysm clipping, arteriovenous malformation resection, or during complex brain surgeries. The aim is to reduce blood loss and allow for better visibility during the surgery.
Step 2: Indications for Circulatory Arrest in Cerebrovascular Surgery:
1. Aneurysm Clipping: In certain cases, especially with complex aneurysms, circulatory arrest is used to control blood flow and prevent rupture during surgery.
2. Arteriovenous Malformations (AVMs): In surgeries involving AVMs, circulatory arrest can help control bleeding and improve surgical access.
3. Neurosurgical Resection: In cases requiring the resection of large tumors or other vascular lesions, circulatory arrest may be employed to enhance visualization and minimize bleeding.
Step 3: Techniques of Circulatory Arrest:
1. Hypothermic Arrest: This involves cooling the patient to induce a state of hypothermia, which reduces metabolic demand and allows the brain to tolerate the lack of blood flow.
2. Warm Arrest: In some cases, a warm arrest is performed where blood flow is temporarily stopped without lowering the body temperature.
3. Selective Circulatory Arrest: This technique focuses on reducing blood flow to specific areas (e.g., the brain or a specific vessel) while maintaining circulation to other areas of the body.
Step 4: Risks and Complications:
1. Ischemia: The most significant risk of circulatory arrest is brain ischemia due to a prolonged lack of oxygenated blood.
2. Neurological Deficits: Depending on the duration of circulatory arrest and the area affected, there may be postoperative neurological deficits.
3. Reperfusion Injury: Upon restoring blood flow, reperfusion injury may occur, leading to inflammation and tissue damage. Quick Tip: Circulatory arrest in neurosurgery offers critical advantages for certain procedures but requires careful monitoring to minimize the risk of ischemic damage.





Comments