Maharashtra Board Class 10 Science and Technology (N934) Question Paper 2023 with Solution PDF pdf is available for download here. The Science and technology paper was conducted on March 13, 2023 in the morning shift from 11:00 AM-2:00 PM. The question paper was divided into two sections - Section A for objective questions and Section B for subjective questions.
| Maharashtra Board Class 10 Science and Technology Question Paper With Solution PDF | Check Solution |

The device used for producing current is called .................................
View Solution
A generator is a device that converts mechanical energy into electrical energy, thereby producing an electric current. This is in contrast to the other devices listed:
- A voltmeter measures the potential difference (voltage) across two points.
- An ammeter measures the current flowing through a circuit.
- A galvanometer detects small currents, typically for indicating presence or magnitude of current. Quick Tip: Remember that devices like generators produce current by converting mechanical energy, while voltmeters, ammeters, and galvanometers are used for measuring electrical quantities.
If a ray of light passes from a denser medium to a rarer medium in a straight line, the angle of incidence must be ................
View Solution
When a ray of light passes from a denser medium (like water or glass) to a rarer medium (like air), if it travels in a straight line without bending, it must be incident at \( 0^\circ \), meaning the ray is parallel to the normal. This is because refraction occurs only when the light ray strikes the interface at an angle other than \( 0^\circ \). Quick Tip: For light to pass through the boundary between two media without bending, the angle of incidence must be \( 0^\circ \), i.e., the ray must be perpendicular to the surface.
The power of a convex lens of focal length \( 20 cm \) is ....................
View Solution
The power \( P \) of a lens is given by the formula: \[ P = \frac{1}{f} \]
where \( f \) is the focal length in meters. For a convex lens with a focal length of \( 20 cm \) (or \( 0.2 m \)): \[ P = \frac{1}{0.2} = 5.0 D. \]
The positive sign indicates it is a converging lens (convex lens). Quick Tip: To calculate the power of a lens, use the formula \( P = \frac{1}{f} \), where \( f \) is the focal length in meters. A positive power indicates a convex lens.
Good conductor of electricity is ............................
View Solution
Among the options, graphite is a good conductor of electricity. This is because graphite has a layered structure, and the layers are held together by weak forces, allowing the electrons to move freely between them, thus conducting electricity. The other materials (bromine, iodine, and sulfur) are poor conductors or insulators. Quick Tip: Graphite is a unique form of carbon that conducts electricity due to its structure, while most non-metals like sulfur, bromine, and iodine do not conduct electricity.
The height of medium earth orbit above the surface of the earth is :
View Solution
The medium Earth orbit (MEO) is typically located between low Earth orbit (LEO) and geostationary orbit (GEO). The most commonly used medium Earth orbit for satellites is at an altitude of approximately \( 250 km \). This is higher than LEO (which is around \( 160-2,000 km \)) but much lower than geostationary orbits (about \( 35,786 km \)). Quick Tip: Medium Earth Orbits (MEO) are generally found at altitudes of about \( 250 km \) to \( 35,000 km \). LEO is closer to the Earth, while GEO is far higher at around \( 36,000 km \).
Find the odd man out: Loudspeaker, Microphone, Electric motor, Magnet.
View Solution
The odd one out is the magnet. A loudspeaker, microphone, and electric motor are all devices that involve the interaction of electromagnetic fields to function. A loudspeaker and microphone convert sound into electrical signals and vice versa, while an electric motor converts electrical energy into mechanical energy using magnetic fields. A magnet, on the other hand, is a passive object that does not rely on electricity or electromagnetism to operate in the same way as the other devices. Quick Tip: Devices like loudspeakers, microphones, and electric motors actively use magnetic fields and electricity to function, while a magnet is a passive object that creates a magnetic field.
Complete the co-relation: \[ CuI_2 : Brown :: AgCl : ............................ \]
View Solution
In this correlation, \(CuI_2\) is brown in color. Now, \(AgCl\) (Silver chloride) is a white precipitate. It is a well-known fact that \(AgCl\) appears white when formed as a precipitate in reactions involving silver ions. Therefore, the correct match for \(AgCl\) is "White." Quick Tip: In inorganic chemistry, the color of precipitates can be an important characteristic for identification. For example, \(CuI_2\) is brown, while \(AgCl\) is white.
Match the pair:
Group ’A’ Group ’B’
Substance Refractive index
Air (a) 1.33
Water (b) 1.46
Glass (c) 1.0003
Air (c) 1.0003
Water (a) 1.33
Glass (b) 1.46
View Solution
- The refractive index of air is approximately \( 1.0003 \), which is very close to 1, as air has almost the same refractive index as a vacuum.
- Water has a refractive index of about \( 1.33 \), meaning light slows down by a factor of \( 1.33 \) when it enters water from air.
- Glass typically has a refractive index of around \( 1.46 \), meaning light travels slower in glass compared to both air and water. Quick Tip: Refractive index is the ratio of the speed of light in a vacuum to the speed of light in the material. The higher the refractive index, the slower light travels in that material.
State True or False: "Wavelength of red light is close to \( 700 nm \)."
View Solution
The wavelength of red light is typically in the range of \( 620 nm \) to \( 750 nm \). The value \( 700 nm \) is a commonly accepted wavelength for red light in the visible spectrum, so the statement is true. Quick Tip: The visible spectrum of light ranges from violet (~400 nm) to red (~700 nm). Red light specifically has wavelengths close to 700 nm, typically ranging between 620 nm and 750 nm.
Write the name of the small satellite made by a group of students from \(COEP\) (College of Engineering, Pune) sent to space through \(ISRO\) in \(2016\).
View Solution
In 2016, a group of students from the College of Engineering, Pune (COEP) developed a small satellite called Pratham. It was India's first student-built nanosatellite, and it was sent to space as part of the Indian Space Research Organisation (ISRO)'s mission. The satellite was used to measure the density of free electrons in Earth's ionosphere. Quick Tip: Student-designed satellites like \textit{Pratham showcase the growing involvement of educational institutions in space exploration, encouraging hands-on learning and innovation in the field of aerospace engineering.
For electric power transmission, copper or aluminium wire is used.
View Solution
Copper and aluminium are both commonly used in electrical power transmission due to their good electrical conductivity. Copper has a slightly better conductivity, but aluminium is lighter and cheaper, which makes it a popular choice for long-distance transmission lines. Quick Tip: Copper and aluminium are favored materials for electrical wiring due to their low resistance and high conductivity. Aluminium, being lighter and cheaper, is often used in overhead power lines.
Lemon or tamarind is used for cleaning copper vessels turned greenish.
View Solution
Lemon or tamarind is acidic in nature and can effectively remove the greenish coating (copper carbonate) that forms on copper vessels due to oxidation. The acid helps in dissolving the copper salts and restoring the vessel’s shine. Quick Tip: Acidic substances like lemon and tamarind are commonly used to clean tarnished copper, as they react with copper oxide and dissolve the greenish coating.
Elements belonging to the same group have the same valency.
View Solution
Elements in the same group of the periodic table have the same number of valence electrons. Since valency is determined by the number of electrons an atom can lose, gain, or share to complete its outer electron shell, elements in the same group exhibit the same valency. For example, all elements in Group 1 (alkali metals) have a valency of 1, and all elements in Group 17 (halogens) have a valency of 1 as well. Quick Tip: The valency of an element is related to the number of valence electrons in its outermost shell. Elements in the same group share the same number of valence electrons, hence they have the same valency.
How do we feel about air in each of the following conditions?
(a) Relative humidity is more than \( 60% \).
(b) Relative humidity is less than \( 60% \).
View Solution
(a) When the relative humidity is more than \( 60% \), the air feels more humid, and we may feel uncomfortable, especially in hot weather. Our body’s ability to cool itself through evaporation (sweating) becomes less efficient, making us feel warmer than the actual temperature.
(b) When the relative humidity is less than \( 60% \), the air feels drier. In such conditions, we may experience dryness in the skin, eyes, and throat, especially in extreme cases. Evaporation is more effective, and we may feel cooler than the actual temperature.
Relative humidity is the amount of water vapor present in the air compared to the maximum amount it can hold at a given temperature.
- In high humidity (more than \( 60% \)), the air retains more moisture, making it harder for sweat to evaporate, thus making us feel hotter and sticky.
- In low humidity (less than \( 60% \)), the air feels dry, and moisture evaporates more quickly from our skin, which can make us feel cooler but also potentially dehydrated. Quick Tip: When relative humidity is high, the air feels warmer because sweat doesn’t evaporate as efficiently, while low humidity makes the air feel cooler but can cause dryness.
Complete the following reaction: \[ C_{12}H_{22}O_{11} \xrightarrow{heat} .................. + .................. \]
View Solution
\[ C_{12}H_{22}O_{11} \xrightarrow{heat} C_{6}H_{12}O_{6} + C_{6}H_{12}O_{6} \]
(Sucrose undergoes hydrolysis to produce glucose and fructose upon heating.)
The given reaction involves the hydrolysis of sucrose (common sugar). When sucrose (\(C_{12}H_{22}O_{11}\)) is heated, it breaks down into two monosaccharides: glucose (\(C_6H_{12}O_6\)) and fructose (\(C_6H_{12}O_6\)). Quick Tip: Heating sucrose (table sugar) can cause it to undergo hydrolysis, where it breaks into glucose and fructose, a process important in food chemistry.
Distinguish between Mass and Weight.
View Solution
Mass: Mass is a measure of the amount of matter in an object. It is a scalar quantity and is typically measured in kilograms (kg) or grams (g). Mass does not change regardless of the object’s location in the universe.
Weight: Weight is the force exerted by gravity on an object. It is a vector quantity and depends on both the mass of the object and the gravitational acceleration. The formula for weight is: \[ Weight = Mass \times Gravitational acceleration \]
Weight varies depending on the location of the object (for example, on Earth, weight is measured in newtons, while it would be less on the Moon).
- Mass is an intrinsic property of matter, meaning it remains constant regardless of the object's location.
- Weight depends on the local gravitational field. For instance, an object weighs less on the Moon due to weaker gravity than it does on Earth. Quick Tip: Mass is always constant, while weight changes with location due to variations in gravitational force.
Complete the following table:

View Solution
Type of Satellite The names of Indian Satellite and launcher
(1) Navigational Satellite Satellite : IRNSS-1A, IRNSS-1B, etc. \> Launcher : \text{PSLV (Polar Satellite Launch Vehicle)
(2) Earth observation Satellite Satellite : RISAT, Cartosat, etc. \> Launcher : \text{PSLV (Polar Satellite Launch Vehicle), GSLV (Geosynchronous Satellite Launch Vehicle)
1. Navigational Satellites are primarily used for positioning, navigation, and timing. India’s navigational satellites belong to the IRNSS (Indian Regional Navigation Satellite System) series, with the PSLV used as the launcher.
2. Earth Observation Satellites are used for remote sensing applications such as weather monitoring, resource management, and disaster monitoring. India has launched several Earth observation satellites such as RISAT and Cartosat using PSLV and GSLV launch vehicles. Quick Tip: India's space missions use a variety of launch vehicles like PSLV and GSLV to deploy different types of satellites, including navigation and Earth observation satellites.
Define periods and groups of the modern periodic table.
View Solution
Periods:
A period is a horizontal row in the periodic table. There are 7 periods in the modern periodic table. The elements in a period have the same number of electron shells or energy levels. As we move across a period from left to right, the atomic number increases, and the properties of elements gradually change.
Groups:
A group is a vertical column in the periodic table. There are 18 groups in the modern periodic table. Elements in the same group have the same number of valence electrons, which gives them similar chemical properties. For example, Group 1 contains the alkali metals, and Group 17 contains the halogens.
- Periods: Periods are horizontal rows in the periodic table. Elements in the same period have the same number of electron shells. As we move from left to right across a period, the elements go from metallic to non-metallic character.
- Groups: Groups are vertical columns in the periodic table. Elements in the same group have similar chemical properties due to having the same number of valence electrons. For example, Group 1 elements are all highly reactive metals, while Group 17 elements (halogens) are all non-metals. Quick Tip: The periodic table is organized into periods (rows) and groups (columns), with elements in the same group exhibiting similar chemical behaviors due to the same number of valence electrons.
Calculate the escape velocity on the surface of the Moon, given the mass and radius of the Moon to be \( 7.34 \times 10^{22} \, kg \) and \( 1.74 \times 10^6 \, m \) respectively. (Given: \( G = 6.67 \times 10^{-11} \, Nm^2/kg^2 \))
View Solution
The escape velocity \( v_e \) is given by the formula: \[ v_e = \sqrt{\frac{2GM}{R}} \]
where:
- \( G \) is the gravitational constant (\( 6.67 \times 10^{-11} \, Nm^2/kg^2 \)),
- \( M \) is the mass of the Moon (\( 7.34 \times 10^{22} \, kg \)),
- \( R \) is the radius of the Moon (\( 1.74 \times 10^6 \, m \)).
Substituting the given values into the formula: \[ v_e = \sqrt{\frac{2 \times 6.67 \times 10^{-11} \times 7.34 \times 10^{22}}{1.74 \times 10^6}} \]
Calculating the numerator: \[ 2 \times 6.67 \times 10^{-11} \times 7.34 \times 10^{22} = 9.78 \times 10^{12} \]
Now, calculating the escape velocity: \[ v_e = \sqrt{\frac{9.78 \times 10^{12}}{1.74 \times 10^6}} = \sqrt{5.63 \times 10^6} \]
\[ v_e \approx 2373 \, m/s \]
Thus, the escape velocity on the surface of the Moon is approximately \( \boxed{2.37 \, km/s} \).
The escape velocity is the minimum velocity needed for an object to escape the gravitational field of a celestial body. The formula for escape velocity is derived from the law of conservation of energy and involves the gravitational constant, mass, and radius of the body.
In this case, by substituting the known values for the Moon's mass and radius into the formula, we find that the escape velocity from the Moon's surface is about \( 2.37 \, km/s \). Quick Tip: The escape velocity depends on the mass and radius of the celestial body. A smaller radius or greater mass results in a higher escape velocity. Escape velocity is independent of the object's mass.
An element has its electron configuration as \(2, 8, 1\). Now answer the following questions:
(a) What is the atomic number of this element?
(b) What is the group of this element?
(c) To which period does the element belong?
View Solution
(a) Atomic number: The atomic number is the total number of electrons in an atom. Given the electron configuration \( 2, 8, 1 \), the total number of electrons is \( 2 + 8 + 1 = 11 \). Thus, the atomic number of the element is \( 11 \).
(b) Group: The group of an element is determined by the number of valence electrons (the electrons in the outermost shell). Since the outermost shell has 1 electron, the element belongs to Group 1, which contains alkali metals.
(c) Period: The period of an element is determined by the number of electron shells. Since the electron configuration has 3 energy levels (or shells), the element belongs to Period 3.
Correct Answer:
(a) The atomic number of the element is \( 2 + 8 + 1 = 11 \).
(b) The element belongs to Group 1, as it has 1 electron in its outermost shell.
(c) The element belongs to Period 3, as the electron configuration has 3 energy levels. Quick Tip: The atomic number is simply the sum of the electrons in an atom. The group is determined by the number of valence electrons, and the period is determined by the number of electron shells.
Observe the figure and name the ray \( AB \), ray \( CD \), ray \( GH \):
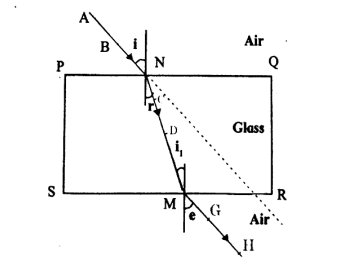
View Solution
Ray \( AB \): This is the incident ray, which is the light ray that approaches the surface of the glass slab and strikes it.
Ray \( CD \): This is the refracted ray, which is the light ray that bends as it enters the glass slab, due to the change in the speed of light when moving from one medium (air) to another (glass).
Ray \( GH \): This is the emergent ray, which is the light ray that exits the glass slab after it has been refracted inside the slab.
The phenomenon of refraction occurs due to the change in the speed of light as it moves from one medium to another with a different refractive index. Quick Tip: In refraction, the incident ray strikes the surface, the refracted ray bends inside the medium, and the emergent ray exits the medium. The change in speed and direction of light when it moves between different media is key to understanding refraction.
Read the following sentence and answer the questions: "\(NaCl\) is an ionic compound."
(a) Why is \( NaCl \) an ionic compound?
(b) State any two properties of ionic compounds.
View Solution
(a) Why is \( NaCl \) an ionic compound?
\( NaCl \) (sodium chloride) is an ionic compound because it is formed when sodium (Na) donates one electron to chlorine (Cl). This results in the formation of \( Na^+ \) (sodium ion) and \( Cl^- \) (chloride ion), which are held together by strong electrostatic forces of attraction, forming an ionic bond.
(b) Properties of Ionic Compounds:
Ionic compounds have high melting and boiling points due to the strong electrostatic forces between the ions.
Ionic compounds conduct electricity in molten or aqueous form because the ions are free to move and carry charge. Quick Tip: Ionic compounds are formed when electrons are transferred from one atom to another, resulting in oppositely charged ions that attract each other. These compounds tend to have high melting points and can conduct electricity when dissolved in water or melted.
Identify the physical and chemical changes from the following phenomena:
(a) Transformation of ice into water.
(b) Ripening of fruit.
(c) Milk turned into curd.
(d) Evaporation of water.
(e) Digestion of food in the stomach.
[(f)] Iron filings get attracted towards the magnet.
View Solution
(a) Transformation of ice into water:
This is a physical change because the substance (H2O) remains the same; only its state changes from solid to liquid.
(b) Ripening of fruit:
This is a chemical change because new substances, such as sugars and acids, are formed as the fruit ripens.
(c) Milk turned into curd:
This is a chemical change because it involves a biochemical process where bacteria ferment milk, converting lactose into lactic acid, which leads to the formation of curd.
(d) Evaporation of water:
This is a physical change because the water only changes from liquid to gas without altering its chemical composition.
(e) Digestion of food in the stomach:
This is a chemical change because food undergoes chemical reactions like hydrolysis, breaking down into simpler compounds.
[(f)] Iron filings get attracted towards the magnet:
This is a physical change because it only changes the magnetic properties of the iron filings, not their chemical structure. Quick Tip: In physical changes, no new substances are formed, and the changes are typically reversible. In chemical changes, new substances are formed, and the process is often irreversible.
Observe the following figure and answer the questions:
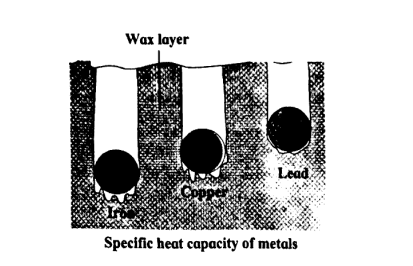
(a) Which element has maximum specific heat capacity? Justify.
(b) Which element has minimum specific heat capacity? Justify.
(c) Define specific heat of an object.
View Solution
(a) Maximum Specific Heat Capacity:
The specific heat capacity is the amount of heat needed to raise the temperature of a substance. From the figure, the wax layer in the test tube containing Lead melts the most slowly. This indicates that lead has a higher specific heat capacity compared to the other metals, as it requires more heat to raise its temperature.
(b) Minimum Specific Heat Capacity:
Iron shows the fastest melting of the wax layer, indicating that it has the lowest specific heat capacity. Iron requires less heat to raise its temperature, so the wax melts quickly compared to the other metals.
(c) Definition of Specific Heat:
The specific heat of an object is defined as the amount of heat energy required to raise the temperature of one kilogram of the substance by one degree Celsius (or one Kelvin). It is usually represented by the symbol \( c \) and has units of \( J/kg^\circ C \) or \( J/kg K \). Quick Tip: A substance with a high specific heat capacity can absorb more heat without a significant rise in temperature, while a substance with a low specific heat capacity will experience a greater temperature change for the same amount of heat added.
Complete the following flow chart:
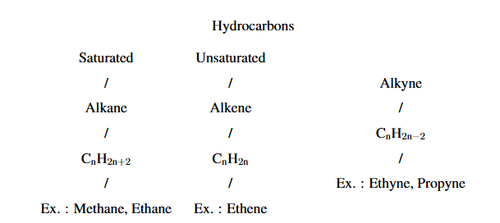
View Solution
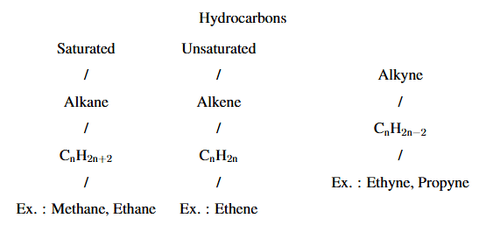
Saturated Hydrocarbons: These contain only single bonds between carbon atoms. They are also known as alkanes and have the general formula \( C_nH_{2n+2} \). Examples include Methane, Ethane, etc.
Unsaturated Hydrocarbons: These contain at least one multiple bond (either a double or triple bond) between carbon atoms:
Alkenes: These have at least one double bond between carbon atoms and follow the general formula \( C_nH_{2n} \). An example is Ethene.
Alkynes: These have at least one triple bond between carbon atoms and follow the general formula \( C_nH_{2n-2} \). Examples include Ethyne (acetylene) and Propyne. Quick Tip: Hydrocarbons are classified into saturated (alkanes) and unsaturated (alkenes and alkynes) based on their bonding. Saturated hydrocarbons have single bonds, while unsaturated ones have double or triple bonds.
Observe the figure and answer the following questions:

(a) Name the defect of vision represented in the above figure.
(b) State the reasons for this defect.
(c) How is it corrected?
(d) Draw the diagram to show the correction of this defect.
View Solution
(a) Name of the defect: The defect of vision shown in the figure is Myopia (or Near-sightedness). In this condition, a person can see nearby objects clearly but has difficulty seeing distant objects because the image of distant objects forms in front of the retina.
(b) Reasons for this defect:
Myopia occurs due to:
An eyeball that is too long.
A lens with too short a focal length.
These factors cause light rays to converge too soon, before reaching the retina.
(c) How is it corrected? Myopia is corrected with concave lenses (lenses that diverge light). These lenses spread the light rays before they enter the eye, allowing the rays to focus correctly on the retina.
(d) Diagram to show the correction of myopia:
[Insert a diagram here showing a concave lens diverging the rays of light before they enter the eye, ensuring the rays focus on the retina.] Quick Tip: Myopia is corrected using concave lenses because these lenses diverge the incoming light rays, shifting the focal point onto the retina. Remember, concave lenses are thinner at the center than at the edges.
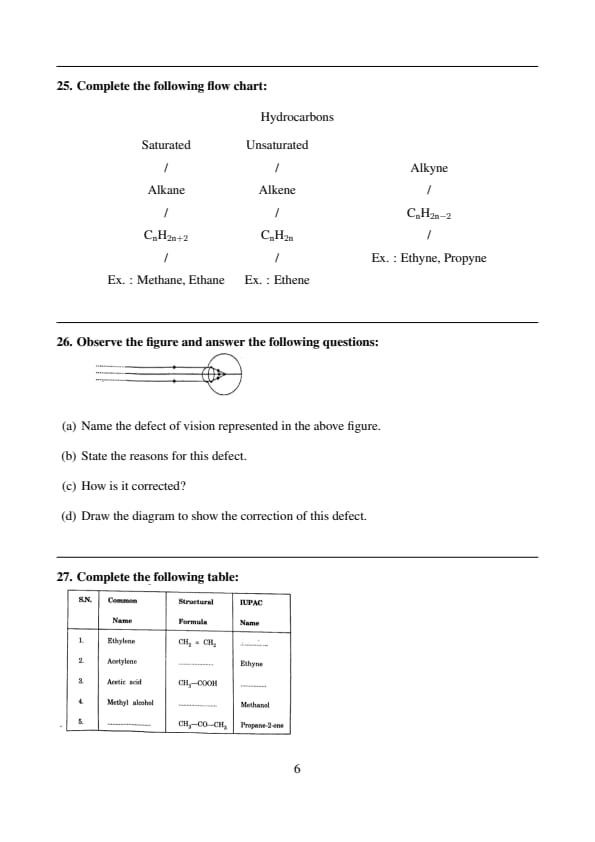
Complete the following table:
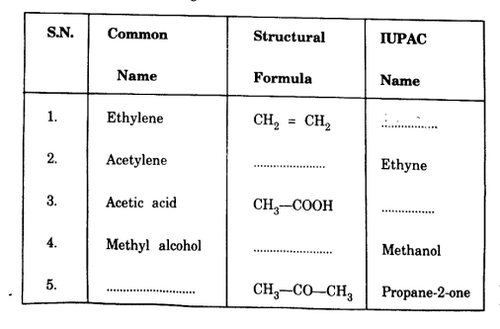
View Solution
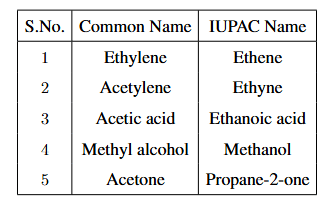
1. Ethylene: The IUPAC name of Ethylene (\(CH_2 = CH_2\)) is Ethene. It is an alkene with two carbon atoms and a double bond between them.
2. Acetylene: The IUPAC name of Acetylene (\(CH_≡CH\)) is Ethyne. It is an alkyne with a triple bond between two carbon atoms.
3. Acetic acid: The IUPAC name of Acetic acid (\(CH_3 - COOH\)) is Ethanoic acid. It is a carboxylic acid with two carbon atoms.
4. Methyl alcohol: The IUPAC name of Methyl alcohol (\(CH_3 - OH\)) is Methanol. It is the simplest alcohol, with one carbon atom and a hydroxyl group (-OH).
5. Acetone: The IUPAC name of Acetone (\(CH_3 - CO - CH_3\)) is Propane-2-one. It is a ketone with three carbon atoms and a carbonyl group (C=O) attached to the second carbon. Quick Tip: When converting from common names to IUPAC names: - Ethylene is an alkene, so its IUPAC name is "Ethene." - Acetylene is an alkyne, so its IUPAC name is "Ethyne." - Acetic acid is a carboxylic acid, so its IUPAC name is "Ethanoic acid." - Methyl alcohol is a simple alcohol, so its IUPAC name is "Methanol." - Acetone is a ketone, and its IUPAC name is "Propane-2-one."












Comments