Maharashtra Board Class 12 Chemistry Question Paper 2023 with Answer Key pdf is available for download here. The exam was conducted by Maharashtra State Board of Secondary & Higher Secondary Education (MSBSHSE) on March 1, 2023 in the Forenoon Session 11 AM to 2 PM. The question paper comprised a total of 31 questions divided among 4 sections.
Maharashtra Board Class 12 Chemistry Question Paper 2023 with Answer Key
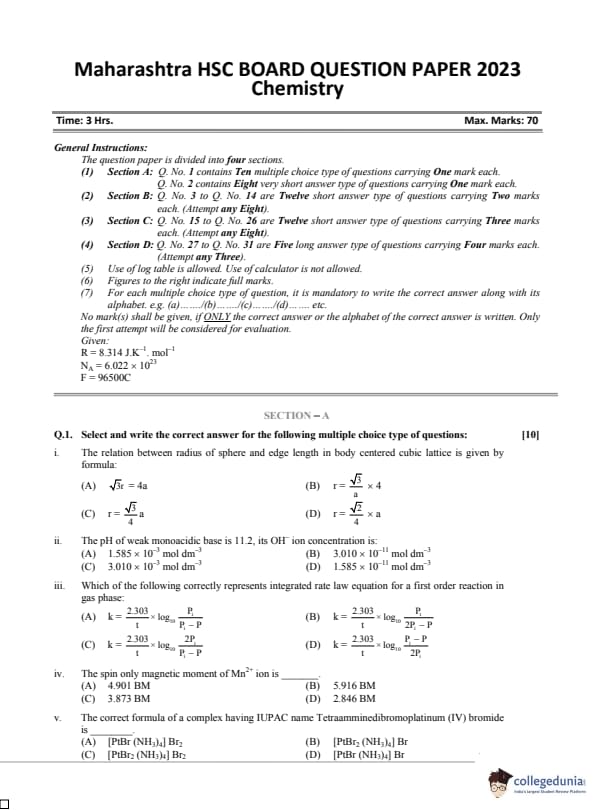
| Maharashtra Board Class 12 Chemistry Question Paper 2023 with Answer Key | Check Solution |

The relation between radius of sphere and edge length in body centered cubic lattice is given by formula:
View Solution
In a body-centered cubic (BCC) lattice, the body diagonal equals √3a, where a is
the edge length. The body diagonal equals 4r (two atomic radii from corner to body center).
Thus:
4r = √3a ⇒ r =
√3
4 a.
The pH of weak monoacidic base is 11.2, its OH⁻ ion concentration is:
View Solution
pH = 11.2, so pOH = 14 − 11.2 = 2.8.
[OH−] = 10−pOH = 10−2.8 = 10−2 × 10−0.8 ≈ 10−2 × 0.1585 = 1.585 × 10−3 mol dm−3.
Which of the following correctly represents integrated rate law equation for a first order reaction in gas phase:
View Solution
For a first-order gas phase reaction A → 2B, initial pressure Pi, pressure of A at
time t = PA, total pressure P = PA + PB . Since PB = 2(Pi − PA), total pressure
P = PA + 2Pi − 2PA = 2Pi − PA. Thus, PA = 2Pi − P . Integrated rate law:
k = 2.303
t log [A]0
[A] = 2.303
t log Pi
2Pi − P .
The spin only magnetic moment of Mn\(^{2+}\) ion is _______.
View Solution
Mn2+ has electron configuration [Ar] 3d5. Number of unpaired electrons n = 5.
Spin-only magnetic moment:
μ = pn(n + 2) = p5(5 + 2) = √35 ≈ 5.916 BM.
The correct formula of a complex having IUPAC name Tetraamminedibromoplatinum (IV) bromide is _______.
View Solution
Tetraamminedibromoplatinum(IV) indicates Pt4+ with 4 NH3 (ammine) and 2 Br−
(bromo) ligands in the coordination sphere, and bromide counter ions balance the +2 charge
of the complex ion. Thus, [PtBr2(NH3)4]2+ with 2 Br− gives [PtBr2(NH3)4]Br2.
The allylic halide, among the following is _______.
View Solution
An allylic halide has a halogen atom on a carbon adjacent to a C=C double bond.
Option (D) CH2 CH CH2 X shows the halogen on the CH2 next to the double
bond, making it allylic.
The product of following reaction is \( \chemfig{CH_3-CH=CH-CH_2-CHO} \xrightarrow{i) LiAlH_4, ii) H_3O^+} \)?
View Solution
LiAlH4 reduces aldehydes to primary alcohols without affecting the C=C double
bond. The aldehyde CHO becomes CH2OH, resulting in
CH3 CH CH CH2 CH2 OH.
Ozonolysis of 2,3-dimethyl but-2-ene, followed by decomposition by Zn dust and water gives _______.
View Solution
2,3-dimethyl but-2-ene is CH32C CCH32 . Ozonolysis cleaves the C=C bond, each
carbon forming a carbonyl. Both carbons have two methyl groups, yielding two molecules of
acetone (CH32C O).
The monomer of natural rubber is _______.
View Solution
Natural rubber is a polymer of isoprene (2-methyl-1,3-butadiene), with repeating units forming polyisoprene. Quick Tip: For chemistry MCQs, focus on key concepts like lattice geometry, pH calculations, reaction mechanisms, and molecular structures to select the correct option efficiently.
Write the name of the technique used to know geometry of nanoparticles.
View Solution
TEM is commonly used to determine the geometry, size, and shape of
nanoparticles by passing electrons through a thin sample to produce high-resolution images
of its structure.
Write the name of the product formed by the action of LiAlH4/ether on acetamide.
View Solution
Acetamide (CH3 C ONH2) is reduced by LiAlH4 in dry ether, converting
the carbonyl group to a methylene group, yielding ethylamine (CH3 CH2 NH2).
Write the structure of the product formed when chlorobenzene is treated with sodium metal in the presence of dry ether.
View Solution
Chlorobenzene (C6H5 Cl) reacts with sodium in dry ether via the Wurtz-Fittig
reaction, coupling two phenyl groups to form biphenyl (C6H5 C6H5).
Write the chemical composition of cryolite.
View Solution
Cryolite is a naturally occurring mineral used in aluminum extraction, with the
chemical formula Na3AlF6 (sodium hexafluoroaluminate).
Write the name of platinum complex used in the treatment of cancer.
View Solution
Cisplatin (cis-[PtCl2(NH3)2]) is a platinum-based coordination complex widely
used as a chemotherapeutic agent for treating various cancers.
Write the correct condition for spontaneity in terms of Gibbs energy.
View Solution
For a process to be spontaneous at constant temperature and pressure, the Gibbs
free energy change must be negative: ∆G = ∆H − T ∆S < 0.
Calculate molar conductivity for 0.5 M BaCl\(_2\) if its conductivity at 298K is 0.01 \( \Omega^{-1} cm^{-1} \).
View Solution
Molar conductivity \( \Lambda_m = \frac{\kappa \cdot 1000}{C} \), where \( \kappa = 0.01 \, \Omega^{-1} cm^{-1} \), \( C = 0.5 \, mol dm^{-3} = 0.5 \, mol L^{-1} = 500 \, mol m^{-3} \). Convert units: \[ \Lambda_m = \frac{0.01 \cdot 1000}{0.5} = \frac{10}{0.5} = 20 \, \Omega^{-1} cm^2 mol^{-1}. \] Quick Tip: For short-answer chemistry questions, ensure precise chemical formulas, unit derivations, and awareness of standard techniques or compounds used in specific contexts.
Distinguish between lanthanides and actinides.
View Solution
Lanthanides and actinides are f-block elements, but they differ as follows:
1. **Electronic Configuration:**
- Lanthanides: Electrons fill the 4f orbitals ([Xe] 4f\(^{1-14}\) 5d\(^{0-1}\) 6s\(^2\)).
- Actinides: Electrons fill the 5f orbitals ([Rn] 5f\(^{1-14}\) 6d\(^{0-1}\) 7s\(^2\)).
2. **Radioactivity:**
- Lanthanides: Mostly non-radioactive (except promethium).
- Actinides: All are radioactive due to unstable nuclei.
3. **Oxidation States:**
- Lanthanides: Predominantly +3, rarely +2 or +4.
- Actinides: Show variable oxidation states (e.g., +3 to +6 for uranium).
4. **Chemical Reactivity:**
- Lanthanides: Less reactive, similar to group 2 metals.
- Actinides: More reactive, form complexes easily due to higher charge density.
Quick Tip: Focus on orbital filling and radioactivity to distinguish lanthanides from actinides effectively.
Calculate the mole fraction of solute, if the vapour pressure of pure benzene at certain temperature is 640 mmHg and vapour pressure of solution of a solute in benzene is 600 mmHg.
View Solution
Using Raoult’s law for a non-volatile solute: \( P = P_0 x_A \), where \( P_0 = 640 \, mmHg \), \( P = 600 \, mmHg \), \( x_A \) is mole fraction of solvent (benzene).
\[ x_A = \frac{P}{P_0} = \frac{600}{640} = 0.9375. \]
Mole fraction of solute \( x_B = 1 - x_A = 1 - 0.9375 = 0.0625 \).
Answer: Mole fraction of solute = 0.0625.
Quick Tip: Raoult’s law applies to non-volatile solutes; mole fractions sum to 1.
Define: Green chemistry. Write two advantages of nanoparticle and nanotechnology.
View Solution
Definition: Green chemistry is the design of chemical products and processes that reduce or eliminate the use and generation of hazardous substances, promoting sustainability and environmental safety.
Advantages of Nanoparticles and Nanotechnology:
1. **Enhanced Reactivity:** Nanoparticles have a high surface area-to-volume ratio, increasing efficiency in catalysis and drug delivery.
2. **Targeted Applications:** Nanotechnology enables precise targeting (e.g., in medicine for cancer treatment), reducing side effects and improving efficacy. Quick Tip: Green chemistry emphasizes eco-friendly processes; nanoparticles excel due to size-dependent properties.
Explain the following terms:
i. Substitutional impurity defect
ii. Interstitial impurity defect
View Solution
i. Substitutional Impurity Defect: Occurs when an atom in a crystal lattice is replaced by a different type of atom. For example, in a silicon crystal, a phosphorus atom may substitute a silicon atom, altering electrical properties (used in doping semiconductors).
ii. Interstitial Impurity Defect: Occurs when a foreign atom occupies an interstitial (empty) site in the lattice, not replacing any host atom. For example, carbon atoms in iron form interstitial defects, increasing hardness in steel. Quick Tip: Substitutional defects involve atom replacement, while interstitial defects involve atoms in lattice voids.
Write the chemical reactions for the following:
i. Chlorobenzene is heated with fuming H\(_2\)SO\(_4\)
ii. Ethyl bromide is heated with silver acetate
View Solution
i. Chlorobenzene with fuming H\(_2\)SO\(_4\):
\[ \chemfig{C_6H_5-Cl} + \chemfig{H_2SO_4} \xrightarrow{heat} \chemfig{C_6H_5-SO_3H} + \chemfig{HCl} \]
Fuming H\(_2\)SO\(_4\) (containing SO\(_3\)) causes sulfonation, forming benzenesulfonic acid.
ii. Ethyl bromide with silver acetate:
\[ \chemfig{CH_3-CH_2-Br} + \chemfig{CH_3COOAg} \xrightarrow{heat} \chemfig{CH_3-CH_2-OCOCH_3} + \chemfig{AgBr} \]
This is a nucleophilic substitution reaction, forming ethyl acetate and silver bromide precipitate. Quick Tip: Aromatic compounds undergo electrophilic substitution with strong reagents; alkyl halides react with silver salts to form esters.
Define: Acidic buffer solution. Write the relationship between solubility and solubility product for PbI\(_2\).
View Solution
Definition: An acidic buffer solution is a mixture of a weak acid and its conjugate base (or salt), maintaining a relatively constant pH (below 7) by resisting changes upon addition of small amounts of acid or base. Example: CH\(_3\)COOH and CH\(_3\)COONa.
Relationship for PbI\(_2\): For PbI\(_2 \rightleftharpoons Pb^{2+} + 2I^-\), let solubility = \( S \, mol L^{-1} \). Then, \( [Pb^{2+}] = S \), \( [I^-] = 2S \). Solubility product: \[ K_{sp} = [Pb^{2+}][I^-]^2 = S \cdot (2S)^2 = 4S^3. \]
Quick Tip: Acidic buffers use weak acid-conjugate base pairs; for sparingly soluble salts, express \( K_{sp} \) in terms of solubility S.
What is the action of the following reagents on ethyl amine:
i. Chloroform and caustic potash
ii. Nitrous acid
View Solution
i. Chloroform and Caustic Potash: Ethyl amine (\( \chemfig{CH_3-CH_2-NH_2} \)) undergoes the carbylamine reaction: \[ \chemfig{CH_3-CH_2-NH_2} + \chemfig{CHCl_3} + 3KOH \xrightarrow{heat} \chemfig{CH_3-CH_2-NC} + 3KCl + 3H_2O. \]
Product: Ethyl isocyanide (foul-smelling).
ii. Nitrous Acid: Ethyl amine reacts with HNO\(_2\) (from NaNO\(_2\) + HCl) to form an alcohol via diazonium ion decomposition: \[ \chemfig{CH_3-CH_2-NH_2} + \chemfig{HNO_2} \to \chemfig{CH_3-CH_2-OH} + N_2 + H_2O. \]
Product: Ethanol. Quick Tip: Primary amines give isocyanides with chloroform/KOH and alcohols with nitrous acid due to diazonium instability.
Calculate standard Gibbs energy change at 25°C for the cell reaction \( Cd(s) + Sn^{2+}(aq) \to Cd^{2+}(aq) + Sn(s) \), \( E^\circ_{Cd} = -0.403 \, V \), \( E^\circ_{Sn} = -0.136 \, V \).
View Solution
Cell: \( Cd | Cd^{2+} || Sn^{2+} | Sn \).
Anode: \( Cd \to Cd^{2+} + 2e^- \), cathode: \( Sn^{2+} + 2e^- \to Sn \).
\( E^\circ_{cell} = E^\circ_{cathode} - E^\circ_{anode} = -0.136 - (-0.403) = 0.267 \, V \).
Number of electrons \( n = 2 \).
Gibbs energy: \( \Delta G^\circ = -n F E^\circ_{cell} \), \( F = 96485 \, C mol^{-1} \), T = 25°C = 298 K (not needed for standard conditions).
\[ \Delta G^\circ = -2 \times 96485 \times 0.267 \approx -51529.59 \, J mol^{-1} = -51.53 \, kJ mol^{-1}. \]
Quick Tip: Use \( \Delta G^\circ = -n F E^\circ \) for electrochemical cells; positive \( E^\circ \) indicates spontaneity (\( \Delta G^\circ < 0 \)).
Write chemical reaction for the preparation of glucose from sucrose. Write structure of D-ribose.
View Solution
i. Preparation of Glucose from Sucrose: Sucrose (\( C_{12}H_{22}O_{11} \)) is hydrolyzed in the presence of dilute acid (e.g., HCl) or enzyme (invertase) to produce glucose and fructose: \[ \chemfig{C_{12}H_{22}O_{11}} + \chemfig{H_2O} \xrightarrow{H^+ or invertase} \chemfig{C_6H_{12}O_6} (glucose) + \chemfig{C_6H_{12}O_6} (fructose). \]
ii. Structure of D-Ribose: D-Ribose is a pentose sugar with the following open-chain structure: \[ \chemfig{HO-CH_2-CH(OH)-CH(OH)-CH(OH)-CH=O} \]
In its cyclic form (furanose), it is: \[ \chemfig{HO-CH_2-[:30](-[:90]OH)-[:-30](-[:90]OH)-[:-150](-[:90]OH)-[:150]O-[:90]} \] Quick Tip: Sucrose hydrolysis yields equal amounts of glucose and fructose; D-ribose is commonly depicted in its furanose ring form in biochemical contexts.
Define Extensive property. Calculate the work done during the expansion of 2 moles of an ideal gas from 10 dm\(^3\) to 20 dm\(^3\) at 298 K in vacuum.
View Solution
Definition: An extensive property is a physical property of a system that depends on the amount or extent of the substance, such as mass, volume, or total energy.
Calculation of Work Done: For an ideal gas expanding in a vacuum (free expansion), external pressure \( P_{ext} = 0 \). Work done \( W = -P_{ext} \Delta V \).
\[ \Delta V = 20 \, dm^3 - 10 \, dm^3 = 10 \, dm^3 = 0.01 \, m^3. \] \[ W = -0 \cdot 0.01 = 0 \, J. \]
Answer: Work done = 0 J. Quick Tip: In free expansion (in vacuum), no work is done since \( P_{ext} = 0 \); extensive properties scale with system size.
Write the reactions for the formation of nylon 6,6 polymer.
View Solution
Nylon 6,6 is a polyamide formed by condensation polymerization of hexamethylenediamine (\( \chemfig{H_2N-(CH_2)_6-NH_2} \)) and adipic acid (\( \chemfig{HOOC-(CH_2)_4-COOH} \)).
\[ n\chemfig{H_2N-(CH_2)_6-NH_2} + n \chemfig{HOOC-(CH_2)_4-COOH} \to \chemfig{[-NH-(CH_2)_6-NH-CO-(CH_2)_4-CO-]_n} + 2n \chemfig{H_2O}. \]
The reaction forms amide bonds, releasing water molecules. Quick Tip: Nylon 6,6 involves condensation of a diamine and a dicarboxylic acid, forming repeating amide linkages.
Draw structures of the following compounds:
i. Chloric acid
ii. Peroxydisulphuric acid
View Solution
i. Chloric Acid (\( HClO_3 \)):
\[ \chemfig{Cl(=[:90]O)(-[:30]OH)(-[:270]O)} \]
Chlorine is bonded to one hydroxyl group and two double-bonded oxygens.
ii. Peroxydisulphuric Acid (\( H_2S_2O_8 \)):
\[ \chemfig{HO-S(=[:90]O)(=[:270]O)-O-O-S(=[:90]O)(=[:270]O)-OH} \]
Contains a peroxide (\( -O-O- \)) bond linking two sulfur atoms, each with two double-bonded oxygens and one hydroxyl group. Quick Tip: Use Lewis structures to confirm oxidation states and bonding in oxoacids; peroxydisulphuric acid has a characteristic peroxide linkage.
Define Osmosis. How will you determine molar mass of non-volatile solute by elevation of boiling point?
View Solution
Definition: Osmosis is the spontaneous movement of solvent molecules through a semi-permeable membrane from a region of lower solute concentration to a region of higher solute concentration, tending to equalize concentrations.
Determination of Molar Mass:
The elevation of boiling point (\( \Delta T_b \)) for a non-volatile solute is given by: \[ \Delta T_b = K_b \cdot m, \]
where \( K_b \) is the molal boiling point elevation constant, and \( m \) is molality (\( mol kg^{-1} \)).
Step 1: Measure the boiling point of the pure solvent (\( T_0 \)) and the solution (\( T \)). Calculate \( \Delta T_b = T - T_0 \).
Step 2: Molality \( m = \frac{n_B}{w_A} \), where \( n_B = \frac{w_B}{M_B} \) (moles of solute, \( w_B \) = mass of solute, \( M_B \) = molar mass of solute), and \( w_A \) = mass of solvent in kg.
\[ \Delta T_b = K_b \cdot \frac{w_B}{M_B \cdot w_A}. \] \[ M_B = \frac{K_b \cdot w_B}{\Delta T_b \cdot w_A}. \]
Step 3: Use known \( K_b \), measured \( \Delta T_b \), \( w_B \), and \( w_A \) to calculate \( M_B \). Quick Tip: Use precise temperature measurements and known \( K_b \) values for accurate molar mass determination.
Convert the following:
i. Ethyl alcohol into ethyl acetate
\textbf{ii.} Phenol into benzene
\textbf{iii.} Diethyl ether into ethyl chloride
View Solution
i. Ethyl Alcohol to Ethyl Acetate:
\[ \chemfig{CH_3-CH_2-OH} + \chemfig{CH_3-COOH} \xrightarrow{H_2SO_4, heat} \chemfig{CH_3-C(=O)-O-CH_2-CH_3} + \chemfig{H_2O}. \]
Ethyl alcohol reacts with acetic acid via esterification to form ethyl acetate.
ii. Phenol to Benzene:
\[ \chemfig{C_6H_5-OH} \xrightarrow{Zn dust, heat} \chemfig{C_6H_6} + \chemfig{ZnO}. \]
Phenol is reduced by heating with zinc dust, removing the hydroxyl group to form benzene.
iii. Diethyl Ether to Ethyl Chloride:
\[ \chemfig{CH_3-CH_2-O-CH_2-CH_3} + \chemfig{HCl} \xrightarrow{ZnCl_2, heat} 2 \chemfig{CH_3-CH_2-Cl} + \chemfig{H_2O}. \]
Diethyl ether reacts with concentrated HCl in the presence of ZnCl\(_2\) to form ethyl chloride. Quick Tip: Choose reagents specific to functional group transformations for efficient organic conversions.
A weak monobasic acid is 10 percent dissociated in 0.05 M solution. What is percent dissociation in 0.15 M solution?
View Solution
For a weak monobasic acid \( HA \rightleftharpoons H^+ + A^- \), the dissociation constant \( K_a = \frac{\alpha^2 C}{1 - \alpha} \), where \( \alpha \) is the degree of dissociation, and \( C \) is concentration.
Step 1: For 0.05 M, \( \alpha = 0.1 \): \[ K_a = \frac{(0.1)^2 \cdot 0.05}{1 - 0.1} = \frac{0.01 \cdot 0.05}{0.9} = \frac{0.0005}{0.9} \approx 5.5556 \times 10^{-4}. \]
Step 2: For 0.15 M, use \( K_a \): \[ 5.5556 \times 10^{-4} = \frac{\alpha^2 \cdot 0.15}{1 - \alpha}. \]
Since \( \alpha \) is small, approximate \( 1 - \alpha \approx 1 \): \[ \alpha^2 \cdot 0.15 \approx 5.5556 \times 10^{-4}, \quad \alpha^2 \approx \frac{5.5556 \times 10^{-4}}{0.15} \approx 3.7037 \times 10^{-3}. \] \[ \alpha \approx \sqrt{3.7037 \times 10^{-3}} \approx 0.06086. \]
Percent dissociation = \( 0.06086 \times 100 \approx 6.09% \). Quick Tip: For weak electrolytes, dilution increases dissociation but percent dissociation decreases with higher concentration due to \( K_a \) constancy.
Explain dehydrohalogenation reaction of 2-chlorobutane. Write use and environmental effect of CFC.
View Solution
Dehydrohalogenation of 2-Chlorobutane: Dehydrohalogenation involves the removal of HX from an alkyl halide using a strong base (e.g., alcoholic KOH) to form an alkene. For 2-chlorobutane (\( \chemfig{CH_3-CH(Cl)-CH_2-CH_3} \)): \[ \chemfig{CH_3-CH(Cl)-CH_2-CH_3} \xrightarrow{KOH (alc.), heat} \chemfig{CH_3-CH=CH-CH_3} + \chemfig{HCl}. \]
Major product (Saytzeff rule): 2-butene (more substituted alkene). Minor product: 1-butene (\( \chemfig{CH_2=CH-CH_2-CH_3} \)).
Use of CFC: Chlorofluorocarbons (CFCs) are used as refrigerants, propellants in aerosols, and solvents due to their stability and low toxicity.
Environmental Effect of CFC: CFCs deplete the ozone layer by releasing chlorine radicals upon UV exposure, catalyzing ozone (O\(_3\)) breakdown, leading to increased UV radiation reaching Earth. Quick Tip: Saytzeff rule favors the more substituted alkene; CFCs are phased out due to ozone depletion.
2000 mmol of an ideal gas expanded isothermally and reversibly from 20 L to 30 L at 300 K, calculate the work done in the process (\( R = 8.314 \, J K^{-1} mol^{-1} \)).
View Solution
For isothermal reversible expansion, work done \( W = -n R T \ln \left( \frac{V_f}{V_i} \right) \).
\( n = 2000 \, mmol = 2 \, mol \), \( T = 300 \, K \), \( V_i = 20 \, L \), \( V_f = 30 \, L \), \( R = 8.314 \, J K^{-1} mol^{-1} \).
\[ W = -2 \cdot 8.314 \cdot 300 \cdot \ln \left( \frac{30}{20} \right) = -2 \cdot 8.314 \cdot 300 \cdot \ln (1.5). \] \[ \ln (1.5) \approx 0.405465, \quad W \approx -2 \cdot 8.314 \cdot 300 \cdot 0.405465 \approx -2022.77 \, J. \]
Answer: Work done \( \approx -2022.77 \, J \). Quick Tip: For isothermal reversible expansion, work depends on the volume ratio; negative sign indicates work done by the system.
What are interstitial compounds? Give the classification of alloys with examples.
View Solution
Interstitial Compounds: These are compounds formed when small atoms (e.g., H, C, N) occupy interstitial sites in the lattice of transition metals (e.g., Fe, Ti). Example: TiC (titanium carbide), where carbon atoms fit in the metal lattice, enhancing hardness.
Classification of Alloys:
1. **Substitutional Alloys:** Atoms of one metal replace atoms of another in the lattice. Example: Brass (Cu and Zn).
2. **Interstitial Alloys:** Small atoms occupy interstitial sites in a metal lattice. Example: Steel (Fe with C).
3. **Intermetallic Alloys:** Specific stoichiometric compounds of metals. Example: Ni\(_3\)Al (used in jet engines). Quick Tip: Interstitial compounds enhance material properties; alloys are classified based on atomic arrangement.
Draw labelled diagram of H\(_2\) – O\(_2\) fuel cell. Write two applications of fuel cell.
View Solution
Diagram:
Reactions: Anode: \( 2H_2 \to 4H^+ + 4e^- \), Cathode: \( O_2 + 4H^+ + 4e^- \to 2H_2O \).
Applications:
1. Power generation in spacecraft (e.g., Apollo missions).
2. Electric vehicle power sources for clean energy. Quick Tip: Fuel cells convert chemical energy directly to electrical energy; H\(_2\)-O\(_2\) cells produce water as a byproduct.
Explain formation of [CoF\(_6\)]\(^{3-}\) complex with respect to:
i. Hybridisation
ii. Magnetic properties
iii. Inner/outer complex
iv. Geometry
View Solution
For [CoF\(_6\)]\(^{3-}\), Co is in +3 oxidation state (\( Co^{3+} \), d\(^6\)).
i. Hybridisation: F\(^-\) is a weak-field ligand, so high-spin complex. Co\(^{3+}\) uses 3d, 4s, and 4p orbitals for d\(^2\)sp\(^3\) hybridisation (outer orbital complex).
ii. Magnetic Properties: d\(^6\) high-spin: 4 unpaired electrons (\( t_{2g}^4 e_g^2 \)). Magnetic moment: \[ \mu = \sqrt{n(n+2)} = \sqrt{4(4+2)} = \sqrt{24} \approx 4.9 \, BM. \]
iii. Inner/Outer Complex: Uses 4s and 4p orbitals, so it’s an outer orbital complex (d\(^2\)sp\(^3\)).
iv. Geometry: Octahedral, as six F\(^-\) ligands coordinate around Co\(^{3+}\). Quick Tip: Weak-field ligands like F\(^-\) cause high-spin complexes; check ligand field strength for hybridisation.
What is Pseudo first order reaction? Derive integrated rate law equation for zero order reaction.
View Solution
Pseudo First Order Reaction: A reaction that is second order (or higher) but appears first order due to one reactant in large excess, keeping its concentration nearly constant. Example: Hydrolysis of ester in water (\( H_2O \) in excess).
Zero Order Integrated Rate Law: For a zero-order reaction \( A \to products \), rate = \( k \).
\[ -\frac{d[A]}{dt} = k. \]
Integrate: \( \int_{[A]_0}^{[A]} d[A] = -k \int_0^t dt \).
\[ [A] - [A]_0 = -kt \quad \Rightarrow \quad [A] = [A]_0 - kt. \] Quick Tip: Pseudo first order simplifies kinetics; zero-order reactions have constant rates, common in catalyzed reactions.
Explain Aldol condensation of ethanal.
View Solution
Aldol condensation involves the reaction of two aldehyde molecules (with \( \alpha \)-H) in the presence of a base to form a \( \beta \)-hydroxy aldehyde, which may dehydrate to an unsaturated aldehyde. For ethanal (\( \chemfig{CH_3-CHO} \)): \[ 2 \chemfig{CH_3-CHO} \xrightarrow{dil. NaOH} \chemfig{CH_3-CH(OH)-CH_2-CHO} \xrightarrow{-H_2O} \chemfig{CH_3-CH=CH-CHO}. \]
Step 1: Enolate ion forms from one ethanal, attacking the carbonyl of another, forming 3-hydroxybutanal (aldol).
Step 2: Dehydration yields crotonaldehyde (\( \chemfig{CH_3-CH=CH-CHO} \)). Quick Tip: Aldol condensation requires \( \alpha \)-hydrogen; dehydration yields conjugated products.
Explain anomalous behaviour of oxygen in group 16 with respect to:
i. Atomicity
ii. Magnetic property
iii. Oxidation state
View Solution
i. Atomicity: Oxygen exists as a diatomic gas (\( O_2 \)), whereas other group 16 elements (S, Se, Te) form polyatomic structures (e.g., S\(_8\)) due to stronger tendency for catenation.
ii. Magnetic Property: O\(_2\) is paramagnetic due to two unpaired electrons in its molecular orbitals (\( \pi^*_{2p} \)), while others like S\(_8\) are diamagnetic.
iii. Oxidation State: Oxygen commonly shows -2 (e.g., H\(_2\)O), rarely +2 (e.g., OF\(_2\)) due to high electronegativity. Others (S, Se, Te) show +4, +6 due to d-orbital availability. Quick Tip: Oxygen’s anomalies stem from its small size, high electronegativity, and lack of d-orbitals.
Write chemical reactions for the following conversions:
i. Acetic acid into acetic anhydride
ii. Acetic acid into ethyl alcohol
Write IUPAC name and structure of methylphenylamine.
View Solution
i. Acetic Acid to Acetic Anhydride:
\[ 2 \chemfig{CH_3-COOH} \xrightarrow{P_2O_5, heat} \chemfig{CH_3-C(=O)-O-C(=O)-CH_3} + \chemfig{H_2O}. \]
P\(_2\)O\(_5\) dehydrates two acetic acid molecules.
ii. Acetic Acid to Ethyl Alcohol:
\[ \chemfig{CH_3-COOH} \xrightarrow{i) LiAlH_4, ii) H_3O^+} \chemfig{CH_3-CH_2-OH}. \]
LiAlH\(_4\) reduces the carboxyl group to a primary alcohol.
Methylphenylamine:
IUPAC Name: N-Methylaniline.
Structure: \( \chemfig{C_6H_5-NH-CH_3} \). Quick Tip: Use strong reducing agents like LiAlH\(_4\) for carboxylic acid reductions; IUPAC names prioritize functional group hierarchy.
Show that, time required for 99.9 percent completion of a first order reaction is three times the time required for 90 percent completion. Give electronic configuration of Gd (Z = 64). Write the name of nano structured material used in car tyres to increase the life of tyres.
View Solution
i. Time for First Order Reaction: For a first-order reaction, the rate law is \( \ln \frac{[A]_0}{[A]} = kt \).
For 90 percent completion, 10% of reactant remains: \( \frac{[A]}{[A]_0} = 0.1 \).
\[ \ln \frac{1}{0.1} = kt_{90%} \quad \Rightarrow \quad kt_{90%} = \ln 10 \approx 2.3026. \]
For 99.9% completion, 0.1% remains: \( \frac{[A]}{[A]_0} = 0.001 \).
\[ \ln \frac{1}{0.001} = kt_{99.9%} \quad \Rightarrow \quad kt_{99.9%} = \ln 1000 \approx 6.9078. \]
Ratio: \[ \frac{t_{99.9%}}{t_{90%}} = \frac{\ln 1000}{\ln 10} = \frac{3 \ln 10}{\ln 10} = 3. \]
Thus, \( t_{99.9%} = 3 t_{90%} \).
ii. Electronic Configuration of Gd (Z = 64): Gadolinium: [Xe] 4f\(^7\) 5d\(^1\) 6s\(^2\).
iii. Nanostructured Material in Car Tyres: Carbon black nanoparticles.
Solution: Carbon black nanoparticles are used in car tyres to enhance durability, improve traction, and increase resistance to wear. Quick Tip: For first-order reactions, use \( \ln \frac{[A]_0}{[A]} = kt \); nanomaterials like carbon black improve mechanical properties in applications.
Derive relationship between \( \Delta H \) and \( \Delta U \) for gaseous reaction. Define: Vulcanization. What is peptide bond?
View Solution
i. Relationship between \( \Delta H \) and \( \Delta U \): Enthalpy \( H = U + PV \). For a gaseous reaction at constant T, \( \Delta H = \Delta U + \Delta (PV) \). Using ideal gas law, \( PV = nRT \), so: \[ \Delta H = \Delta U + \Delta (n_g RT) = \Delta U + \Delta n_g RT, \]
where \( \Delta n_g \) is the change in moles of gas, \( R = 8.314 \, J K^{-1} mol^{-1} \), T is temperature in K.
ii. Vulcanization: The process of heating natural rubber with sulfur to form cross-links between polymer chains, improving elasticity, strength, and durability.
iii. Peptide Bond: A covalent bond (\( -CO-NH- \)) formed between the carboxyl group of one amino acid and the amino group of another, with loss of water, linking amino acids in proteins. Quick Tip: Use \( \Delta n_g \) to relate \( \Delta H \) and \( \Delta U \); vulcanization enhances rubber properties; peptide bonds are amide linkages in proteins.
Silver crystallizes in fcc structure. If edge length of unit cell is 400 pm, calculate density of silver (Atomic mass of Ag = 108). Write a note on Haloform reaction.
View Solution
i. Density of Silver: For FCC, number of atoms per unit cell \( Z = 4 \). Edge length \( a = 400 \, pm = 4 \times 10^{-8} \, cm \), atomic mass \( M = 108 \, g mol^{-1} \), Avogadro’s number \( N_A = 6.022 \times 10^{23} \, mol^{-1} \).
Density \( \rho = \frac{Z \cdot M}{N_A \cdot a^3} \).
\[ a^3 = (4 \times 10^{-8})^3 = 6.4 \times 10^{-23} \, cm^3. \] \[ \rho = \frac{4 \cdot 108}{6.022 \times 10^{23} \cdot 6.4 \times 10^{-23}} \approx \frac{432}{3.85408 \times 10^1} \approx 11.21 \, g cm^{-3}. \]
ii. Haloform Reaction: Compounds with a methyl ketone group (\( \chemfig{CH_3-C(=O)-} \)) or acetaldehyde, when treated with halogens (e.g., I\(_2\)) and a base (e.g., NaOH), form haloforms (e.g., \( \chemfig{CHI_3} \)) and a carboxylate. Example: \[ \chemfig{CH_3-CO-CH_3} + 3I_2 + 4NaOH \to \chemfig{CHI_3} + \chemfig{CH_3COONa} + 3NaI + 3H_2O. \]
Used in iodoform test to detect methyl ketones or acetaldehyde. Quick Tip: FCC density uses \( Z = 4 \); haloform reaction is diagnostic for compounds with \( CH_3CO- \).
Write Dow process for preparation of Phenol. What is the action of bromine water on phenol? Give reason: Group 16th elements have lower ionisation enthalpy compared to group 15th elements. Write two uses of dioxygen.
View Solution
i. Dow Process for Phenol:
\[ \chemfig{C_6H_5-Cl} \xrightarrow{NaOH, 350°C, high pressure} \chemfig{C_6H_5-ONa} \xrightarrow{HCl} \chemfig{C_6H_5-OH} + \chemfig{NaCl}. \]
Chlorobenzene reacts with aqueous NaOH to form sodium phenoxide, which is acidified to yield phenol.
ii. Action of Bromine Water on Phenol: Phenol reacts with Br\(_2\) in water to form 2,4,6-tribromophenol (white precipitate): \[ \chemfig{C_6H_5-OH} + 3\chemfig{Br_2} \to \chemfig{C_6H_2(Br)_3-OH} + 3\chemfig{HBr}. \]
iii. Reason for Lower Ionisation Enthalpy in Group 16: Group 16 elements (e.g., O, S) have \( np^4 \) configuration, with paired electrons in one p-orbital, causing electron-electron repulsion, making it easier to remove an electron compared to group 15’s stable half-filled \( np^3 \) configuration.
iv. Uses of Dioxygen:
1. Respiration in living organisms.
2. Combustion and industrial processes (e.g., steel production). Quick Tip: Dow process uses high temperature/pressure; phenol’s high reactivity with Br\(_2\) is due to ring activation; group 16’s paired electrons lower ionisation energy.






Comments