The freezing point of the 0.05 molal solution of non - electrolyte in water is? (kf = 1.86)
Solution and Explanation
ΔTf = Kf x m
Given: Kf = 1.86 (in units of molal per degree Celsius)
m = 0.05 molal
Substituting the given values into the formula, we get:
ΔTf = 1.86 x 0.05
ΔTf = 0.093oC
The freezing point depression (ΔTf) is 0.093oC
Freezing point of the solution = Freezing point of water - ΔTf
Freezing point of the solution = 0oC - 0.093oC = -0.093oC
Freezing point of the 0.05 molal solution of the non-electrolyte in water is approximately -0.093oC.
freezing point (ΔTf) can be calculated using the formula ΔTf=kf×m, where kf is the cryoscopic constant and m is the molality. For the given values kf =1.86 and m=0.05, the calculation yields ΔTf=0.093.
Thus, the final freezing point (Tf) is found by subtracting 0.0930.093 from the initial temperature,
Top Questions on Solutions
- We are given with three NaCl samples and their Van't Hoff factors. Which is correct option ?
Sample Van't Haff Factor Sample - 1 (0.1 M) \(i_1\) Sample - 2 (0.01 M) \(i_2\) Sample - 3 (0.001 M) \(i_2\) - Which of the following solutions shows positive deviation from Raoult's law?
- IUPAC name of compound
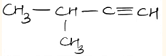
- A saturated \(CaCO_3 \)stock solution is existing at 25°C. In one experiment (i) 25 g \(Na_2CO_3 \)is added to the stock solution. In another experiment (ii) 25 g \(Na_2SO_4\) is added to the stock solution. Select the correct statement from the following.
- The ionic strength of a solution containing 0.01M of $CaCl_2$ and 0.001M of $Na_2SO_4$ is _____ M (rounded off to 3 decimal places)
Questions Asked in BITSAT exam
- Minimum value of 5cos(2x) + 5sin(2x)
- BITSAT - 2023
- Trigonometric Functions
NaOH is deliquescent
- BITSAT - 2023
- States of matter
- Two capacitors, of capacitance C, are connected in series. If one of them is filled with a dielectric substance K, what is the effective capacitance?
- BITSAT - 2023
- electrostatic potential and capacitance
- What is the dimension of the Gravitational constant?
- BITSAT - 2023
- Gravitation
- Oxidation state of S in H2S03 and H2S08
- BITSAT - 2023
- Redox reactions
Concepts Used:
Concentration of Solutions
It is the amount of solute present in one liter of solution.
Concentration in Parts Per Million - The parts of a component per million parts (106) of the solution.
Mass Percentage - When the concentration is expressed as the percent of one component in the solution by mass it is called mass percentage (w/w).
Volume Percentage - Sometimes we express the concentration as a percent of one component in the solution by volume, it is then called as volume percentage
Mass by Volume Percentage - It is defined as the mass of a solute dissolved per 100mL of the solution.
Molarity - One of the most commonly used methods for expressing the concentrations is molarity. It is the number of moles of solute dissolved in one litre of a solution.
Molality - Molality represents the concentration regarding moles of solute and the mass of solvent.
Normality - It is the number of gram equivalents of solute present in one liter of the solution and it is denoted by N.
Formality - It is the number of gram formula present in one litre of solution.



