NaOH is deliquescent
NaOH is deliquescent
Solution and Explanation
That statement is incorrect. Sodium hydroxide (NaOH) is not deliquescent. Deliquescence refers to the property of a substance to absorb moisture from the surrounding environment and dissolve in it, forming a solution.
While sodium hydroxide is a highly hygroscopic compound, meaning it readily absorbs moisture from the atmosphere, it does not dissolve completely in the absorbed water. Instead, it forms a concentrated solution. NaOH is commonly used as a desiccant, meaning it can absorb moisture and remove water vapor from the air. This property makes it useful in various applications such as drying gases and controlling humidity.
Top Questions on States of matter
- A closed vessel contains 10 g of an ideal gas X at 300 K, which exerts 2 atm pressure. At the same temperature, 80 g of another ideal gas Y is added to it and the pressure becomes 6 atm. The ratio of root mean square velocities of X and Y at 300 K is
- JEE Advanced - 2024
- Chemistry
- States of matter
- Number of molecules and moles in 2.8375 litre of O2 at STP.
- JEE Main - 2023
- Chemistry
- States of matter
- Which of the following is a physical change?
- NATA - 2023
- Chemistry
- States of matter
Intermolecular forces are forces of attraction and repulsion between interacting paiticles that will include :
A. dipole - dipole forces.B. dipole - induced dipole forces.C. hydrogen bonding.D. covalent bonding.E. dispersion forces.Choose the most appropriate answer from the options given below :- NEET (UG) - 2023
- Chemistry
- States of matter
- Based on the given figure, the number of correct statement/s is/are _______
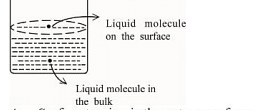
A. Surface tension is the outcome of equal attractive and repulsive forces acting on the liquid molecule in bulk
B. Surface tension is due to uneven forces acting on the molecules present on the surface
C. The molecule in the bulk can never come to the liquid surface
D. The molecules on the surface are responsible for vapour pressure if the system is a closed system- JEE Main - 2023
- Chemistry
- States of matter
Questions Asked in BITSAT exam
- Minimum value of 5cos(2x) + 5sin(2x)
- BITSAT - 2023
- Trigonometric Functions
- Two capacitors, of capacitance C, are connected in series. If one of them is filled with a dielectric substance K, what is the effective capacitance?
- BITSAT - 2023
- electrostatic potential and capacitance
- The freezing point of the 0.05 molal solution of non - electrolyte in water is? (kf = 1.86)
- BITSAT - 2023
- Solutions
- What is the dimension of the Gravitational constant?
- BITSAT - 2023
- Gravitation
- Oxidation state of S in H2S03 and H2S08
- BITSAT - 2023
- Redox reactions
Concepts Used:
States of Matter
The matter is made up of very tiny particles and these particles are so small that we cannot see them with naked eyes.
There are three States of Matter:
The three states of matter are as follows:
Solid State:
- The solid-state is one of the fundamental states of matter.
- Solids differ from liquids and gases by the characteristic of rigidity.
- The molecules of solids are tightly packed because of strong intermolecular forces; they only oscillate about their mean positions.
Liquid State:
- The molecules in a liquid are closely packed due to weak intermolecular forces.
- These forces are weaker than solids but stronger than that of gases.
- There is much space in between the molecules of liquids which makes their flowing ability easy.
Gaseous State:
- In this state of matter, distances between the molecules are large (intermolecular distance is in the range of 10-7-10-5 cm.
- The intermolecular forces experienced between them are negligible.
- Thus, translatory, rotatory and vibratory motions are observed prominently in gases.



